当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Effect of Copper Loading on Iron Carbide Formation and Surface Species in Iron‐Based Fischer–Tropsch Synthesis Catalysts
ChemCatChem ( IF 3.8 ) Pub Date : 2018-02-06 , DOI: 10.1002/cctc.201701673 Diego Peña 1 , Lise Jensen 1 , Andrea Cognigni 1 , Rune Myrstad 2 , Thomas Neumayer 1 , Wouter van Beek 3 , Magnus Rønning 1
ChemCatChem ( IF 3.8 ) Pub Date : 2018-02-06 , DOI: 10.1002/cctc.201701673 Diego Peña 1 , Lise Jensen 1 , Andrea Cognigni 1 , Rune Myrstad 2 , Thomas Neumayer 1 , Wouter van Beek 3 , Magnus Rønning 1
Affiliation
|
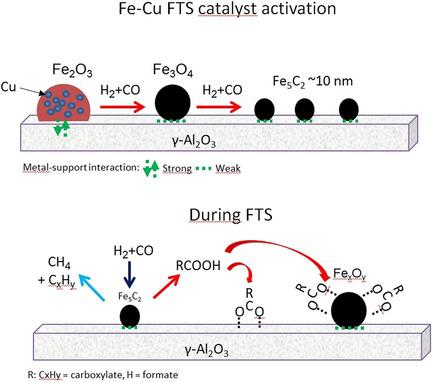
The effect of copper as promoter on iron carbide formation and the nature of surface species on iron‐based catalyst during Fischer–Tropsch synthesis (FTS) was investigated. Iron‐based catalysts (15 wt % of Fe) supported on alumina promoted with copper (0, 0.6, 2, and 5 wt %) were characterised in situ at relevant FTS conditions. The catalysts promoted with 2 and 5 wt % of Cu showed higher catalytic activity due to the formation of Hägg carbide (Fe5C2) detected by in situ XANES and XRD. The carbide formation is attributed to a weakening of the iron–alumina mixed‐compound interactions, and hence increasing iron reducibility and dispersion. The in situ XANES measurements indicated a maximum carburization degree (ca. 20–22 %) even at high Cu loading. The catalyst promoted with 5 wt % of Cu exhibited higher water‐gas shift activity. Aliphatic hydrocarbons, formate, and carboxylate species were detected on the catalyst surface during FTS. After exposing the spent catalysts to hydrogenation conditions, the carboxylate species remained strongly adsorbed while aliphatic hydrocarbons and formate species disappeared. The accumulation of oxygenates species on the catalyst surface increased with Cu loading. The interaction of oxygenates with alumina and iron oxide particles (FexOy) were revealed, with the latter being a possible reason for inhibition of further iron carburization.
中文翻译:

铜负载对铁基费-托合成催化剂中碳化铁形成和表面物种的影响
研究了费-托合成(FTS)过程中铜作为助催化剂对碳化铁形成的影响以及铁基催化剂表面种类的性质。在相关的FTS条件下原位表征了负载在以铜(0、0.6、2和5 wt%)促进的氧化铝上的铁基催化剂(Fe的15 wt%)。由于形成了Hägg碳化物(Fe 5 C 2)通过原位XANES和XRD检测。碳化物的形成归因于铁-氧化铝混合-化合物相互作用的减弱,因此增加了铁的还原性和分散性。原位XANES测量结果表明,即使在高Cu负载下,渗碳度也最高(约20–22%)。以5 wt%的Cu促进的催化剂表现出更高的水煤气变换活性。在FTS期间,在催化剂表面检测到脂肪族烃,甲酸盐和羧酸盐类。在将废催化剂暴露于氢化条件后,羧酸酯物质保持强烈吸附,而脂肪烃和甲酸酯物质消失。含氧化合物在催化剂表面的积累随铜的加入而增加。含氧化合物与氧化铝和氧化铁颗粒(Fe x揭示了O y),后者可能是抑制进一步铁渗碳的可能原因。
更新日期:2018-02-06
中文翻译:

铜负载对铁基费-托合成催化剂中碳化铁形成和表面物种的影响
研究了费-托合成(FTS)过程中铜作为助催化剂对碳化铁形成的影响以及铁基催化剂表面种类的性质。在相关的FTS条件下原位表征了负载在以铜(0、0.6、2和5 wt%)促进的氧化铝上的铁基催化剂(Fe的15 wt%)。由于形成了Hägg碳化物(Fe 5 C 2)通过原位XANES和XRD检测。碳化物的形成归因于铁-氧化铝混合-化合物相互作用的减弱,因此增加了铁的还原性和分散性。原位XANES测量结果表明,即使在高Cu负载下,渗碳度也最高(约20–22%)。以5 wt%的Cu促进的催化剂表现出更高的水煤气变换活性。在FTS期间,在催化剂表面检测到脂肪族烃,甲酸盐和羧酸盐类。在将废催化剂暴露于氢化条件后,羧酸酯物质保持强烈吸附,而脂肪烃和甲酸酯物质消失。含氧化合物在催化剂表面的积累随铜的加入而增加。含氧化合物与氧化铝和氧化铁颗粒(Fe x揭示了O y),后者可能是抑制进一步铁渗碳的可能原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号