当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
From 2- to 3-Substituted Ferrocene Carboxamides or How to Apply Halogen “Dance” to the Ferrocene Series
Organometallics ( IF 2.5 ) Pub Date : 2017-12-01 00:00:00 , DOI: 10.1021/acs.organomet.7b00659 Mehdi Tazi 1 , William Erb 1 , Yury S. Halauko 2 , Oleg A. Ivashkevich 2 , Vadim E. Matulis 3 , Thierry Roisnel 4 , Vincent Dorcet 4 , Florence Mongin 1
Organometallics ( IF 2.5 ) Pub Date : 2017-12-01 00:00:00 , DOI: 10.1021/acs.organomet.7b00659 Mehdi Tazi 1 , William Erb 1 , Yury S. Halauko 2 , Oleg A. Ivashkevich 2 , Vadim E. Matulis 3 , Thierry Roisnel 4 , Vincent Dorcet 4 , Florence Mongin 1
Affiliation
|
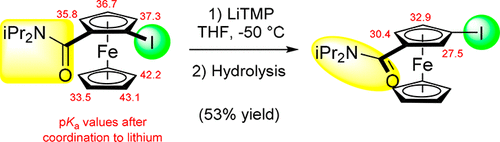
Two methods were compared to convert ferrocene into N,N-diisopropylferrocenecarboxamide, N,N-diethylferrocenecarboxamide, N,N-dimethylferrocenecarboxamide, and (4-morpholinocarbonyl)ferrocene, namely, deprotometalation followed by trapping using dialkylcarbamoyl chlorides and amide formation from the intermediate carboxylic acid. The four ferrocenecarboxamides were functionalized at C2; in the case of the less hindered and more sensitive amides, recourse to a mixed lithium–zinc 2,2,6,6-tetramethylpiperidino-based base allowed us to achieve the reactions. Halogen migration using lithium amides was next optimized. Whereas it appeared impossible to isolate the less hindered 3-iodoferrocenecarboxamides, 3-iodo-N,N-diisopropylferrocenecarboxamide proved stable and was converted to new 1,3-disubstituted ferrocenes by Suzuki coupling or amide reduction. DFT calculations were used to rationalize the results obtained.
中文翻译:

从2到3取代的二茂铁羧酰胺或如何在二茂铁系列中应用卤素“舞蹈”
两种方法进行比较,以转换成二茂铁Ñ,Ñ -diisopropylferrocenecarboxamide,Ñ,Ñ -diethylferrocenecarboxamide,Ñ,Ñ -dimethylferrocenecarboxamide,和(4-吗啉代)二茂铁,即deprotometalation随后从中间羧酸捕集使用二烷基氨基甲氯化物和酰胺形成酸。四种二茂铁羧酰胺在C 2官能化; 在酰胺受阻较少且较敏感的情况下,采用基于混合的锂锌2、2、6、6-四甲基哌啶子基的碱,可以实现反应。接下来优化了使用酰胺化锂的卤素迁移。尽管似乎不可能分离受阻较少的3-碘二茂铁羧酰胺,但是3-碘-N,N-二异丙基二茂铁羧酰胺被证明是稳定的,并通过Suzuki偶联或酰胺还原转化为新的1,3-二取代的二茂铁。DFT计算用于合理化获得的结果。
更新日期:2017-12-01
中文翻译:

从2到3取代的二茂铁羧酰胺或如何在二茂铁系列中应用卤素“舞蹈”
两种方法进行比较,以转换成二茂铁Ñ,Ñ -diisopropylferrocenecarboxamide,Ñ,Ñ -diethylferrocenecarboxamide,Ñ,Ñ -dimethylferrocenecarboxamide,和(4-吗啉代)二茂铁,即deprotometalation随后从中间羧酸捕集使用二烷基氨基甲氯化物和酰胺形成酸。四种二茂铁羧酰胺在C 2官能化; 在酰胺受阻较少且较敏感的情况下,采用基于混合的锂锌2、2、6、6-四甲基哌啶子基的碱,可以实现反应。接下来优化了使用酰胺化锂的卤素迁移。尽管似乎不可能分离受阻较少的3-碘二茂铁羧酰胺,但是3-碘-N,N-二异丙基二茂铁羧酰胺被证明是稳定的,并通过Suzuki偶联或酰胺还原转化为新的1,3-二取代的二茂铁。DFT计算用于合理化获得的结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号