当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dioxygen in Polyoxometalate Mediated Reactions
Chemical Reviews ( IF 51.4 ) Pub Date : 2017-12-01 00:00:00 , DOI: 10.1021/acs.chemrev.7b00444 Ira A. Weinstock 1 , Roy E. Schreiber 2 , Ronny Neumann 2
Chemical Reviews ( IF 51.4 ) Pub Date : 2017-12-01 00:00:00 , DOI: 10.1021/acs.chemrev.7b00444 Ira A. Weinstock 1 , Roy E. Schreiber 2 , Ronny Neumann 2
Affiliation
|
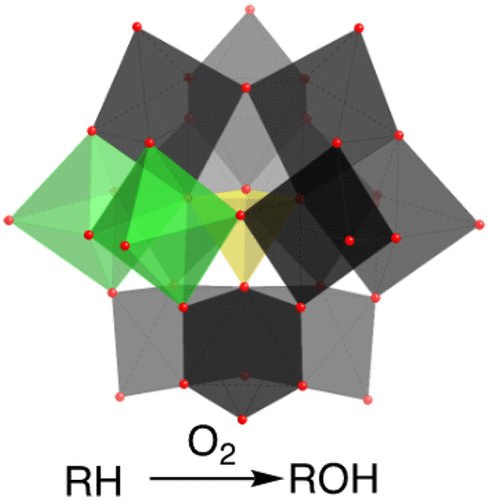
In this review article, we consider the use of molecular oxygen in reactions mediated by polyoxometalates. Polyoxometalates are anionic metal oxide clusters of a variety of structures that are soluble in liquid phases and therefore amenable to homogeneous catalytic transformations. Often, they are active for electron transfer oxidations of a myriad of substrates and upon reduction can be reoxidized by molecular oxygen. For example, the phosphovanadomolybdate, H5PV2Mo10O40, can oxidize Pd(0) thereby enabling aerobic reactions catalyzed by Pd and H5PV2Mo10O40. In a similar vein, polyoxometalates can stabilize metal nanoparticles, leading to additional transformations. Furthermore, electron transfer oxidation of other substrates such as halides and sulfur-containing compounds is possible. More uniquely, H5PV2Mo10O40 and its analogues can mediate electron transfer-oxygen transfer reactions where oxygen atoms are transferred from the polyoxometalate to the substrate. This unique property has enabled correspondingly unique transformations involving carbon–carbon, carbon–hydrogen, and carbon–metal bond activation. The pathway for the reoxidation of vanadomolybdates with O2 appears to be an inner-sphere reaction, but the oxidation of one-electron reduced polyoxotungstates has been shown through intensive research to be an outer-sphere reaction. Beyond electron transfer and electron transfer–oxygen transfer aerobic transformations, there a few examples of apparent dioxygenase activity where both oxygen atoms are donated to a substrate.
中文翻译:

多金属氧酸盐中的双氧介导的反应
在这篇综述文章中,我们考虑在多金属氧酸盐介导的反应中使用分子氧。多金属氧酸盐是具有多种结构的阴离子金属氧化物簇,它们可溶于液相,因此适于均相催化转化。通常,它们对无数种底物的电子转移氧化具有活性,还原后可被分子氧重新氧化。例如,磷钒钼钼酸盐H 5 PV 2 Mo 10 O 40可以氧化Pd(0),从而实现Pd和H 5 PV 2 Mo 10 O 40催化的需氧反应。。同样,多金属氧酸盐可以稳定金属纳米颗粒,从而导致其他转化。此外,其他基底如卤化物和含硫化合物的电子转移氧化也是可能的。更独特的是,H 5 PV 2 Mo 10 O 40及其类似物可以介导电子转移-氧转移反应,其中氧原子从多金属氧酸盐转移至基质。这种独特的性质使得相应的独特转化涉及碳-碳,碳-氢和碳-金属键的活化。O 2还原钒钼酸盐的途径似乎是一个内球反应,但是经过大量研究表明,单电子还原的聚氧钨酸盐的氧化是一个外球反应。除了电子转移和电子转移-氧转移有氧转化之外,还有一些表观双加氧酶活性的例子,其中两个氧原子都被捐赠给了底物。
更新日期:2017-12-01
中文翻译:

多金属氧酸盐中的双氧介导的反应
在这篇综述文章中,我们考虑在多金属氧酸盐介导的反应中使用分子氧。多金属氧酸盐是具有多种结构的阴离子金属氧化物簇,它们可溶于液相,因此适于均相催化转化。通常,它们对无数种底物的电子转移氧化具有活性,还原后可被分子氧重新氧化。例如,磷钒钼钼酸盐H 5 PV 2 Mo 10 O 40可以氧化Pd(0),从而实现Pd和H 5 PV 2 Mo 10 O 40催化的需氧反应。。同样,多金属氧酸盐可以稳定金属纳米颗粒,从而导致其他转化。此外,其他基底如卤化物和含硫化合物的电子转移氧化也是可能的。更独特的是,H 5 PV 2 Mo 10 O 40及其类似物可以介导电子转移-氧转移反应,其中氧原子从多金属氧酸盐转移至基质。这种独特的性质使得相应的独特转化涉及碳-碳,碳-氢和碳-金属键的活化。O 2还原钒钼酸盐的途径似乎是一个内球反应,但是经过大量研究表明,单电子还原的聚氧钨酸盐的氧化是一个外球反应。除了电子转移和电子转移-氧转移有氧转化之外,还有一些表观双加氧酶活性的例子,其中两个氧原子都被捐赠给了底物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号