当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective total synthesis of periconiasin A†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-12-01 00:00:00 , DOI: 10.1039/c7qo00952f Zhixiong Zeng 1, 2, 3, 4, 5 , Cheng Chen 1, 2, 3, 4, 5 , Yandong Zhang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-12-01 00:00:00 , DOI: 10.1039/c7qo00952f Zhixiong Zeng 1, 2, 3, 4, 5 , Cheng Chen 1, 2, 3, 4, 5 , Yandong Zhang 1, 2, 3, 4, 5
Affiliation
|
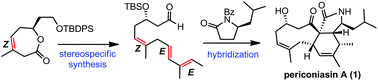
A concise, enantioselective total synthesis of periconiasin A, a hybrid natural product, has been achieved, featuring a ring closing metathesis (RCM) reaction and a Suzuki coupling to secure a (Z,E,E)-skipped triene system and an intramolecular Diels–Alder (IMDA) reaction to construct the challenging 6–9 fused ring system.
中文翻译:

对映体全合成periconiasin A †
一个简单的,对映体选择性的periconiasin A的全合成,一种杂合的天然产物,具有闭环易位(RCM)反应和Suzuki偶联,可确保(Z,E,E)跳过的三烯系统和分子内狄尔斯–Alder(IMDA)反应可构建具有挑战性的6–9稠环系统。
更新日期:2017-12-01
中文翻译:

对映体全合成periconiasin A †
一个简单的,对映体选择性的periconiasin A的全合成,一种杂合的天然产物,具有闭环易位(RCM)反应和Suzuki偶联,可确保(Z,E,E)跳过的三烯系统和分子内狄尔斯–Alder(IMDA)反应可构建具有挑战性的6–9稠环系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号