当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Reversible, Charge-Induced Intramolecular C4a-S-Cysteinyl-Flavin in Choline Oxidase Variant S101C
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1021/acs.biochem.7b00958 Dan Su 1 , Hongling Yuan 1 , Giovanni Gadda 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1021/acs.biochem.7b00958 Dan Su 1 , Hongling Yuan 1 , Giovanni Gadda 1
Affiliation
|
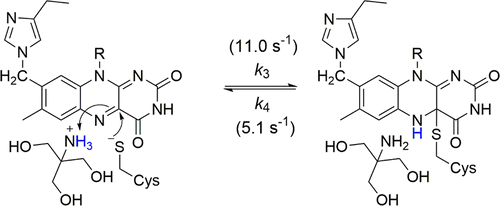
Choline oxidase serves as a paradigm for alcohol oxidation catalyzed by flavin-dependent enzymes. In its active site, S101 is 4 Å from the flavin C4a atom on an extended loop. Enzyme variants substituted at S101 were generated in a previous study and investigated mechanistically [Yuan, H., and Gadda, G. (2011) Biochemistry50, 770–779]. In this study, the typical ultraviolet–visible (UV–vis) absorption spectrum of oxidized flavin was observed for the S101C enzyme in HEPES, TES, or sodium phosphate, whereas an absorption spectrum suggesting the presence of a C4a-flavin adduct with cysteine was obtained in Tris-HCl at pH 8.0. pH titrations of the UV–vis absorption spectrum of the wild-type, S101A, S101C, and H99N enzymes in the presence and absence of Tris allowed for the determination of two pKa values that define a pH range in which the C4a-S-cysteinyl flavin is stabilized. Inhibition studies and stopped-flow kinetics demonstrated that binding of protonated Tris in the active site of the S101C enzyme is required to form the C4a-S-cysteinyl flavin. Deuterium kinetic isotope effects and proton inventories of the S101C enzyme mixed in a stopped-flow spectrophotometer with Tris established a mechanism for the reversible formation of the C4a-S-cysteinyl flavin. This study provides a detailed mechanistic analysis of the reversible formation of a bicovalent C4a-S-cysteinyl-8α-N3-histidyl flavin in choline oxidase, identifying an optimal pH range and a mechanistic rationale for the stabilization of de novo C4a-S-cysteinyl-flavins. Moreover, it presents an example of an intramolecular reaction of an enzyme-bound flavin without a substrate.
中文翻译:

可逆的,电荷诱导的胆碱氧化酶变体S101C中的分子内C4a-S-半胱氨酰-黄素
胆碱氧化酶用作黄素依赖性酶催化的醇氧化的范例。在其活性位点,S101在延伸的环上与黄素C4a原子相距4Å。酶变体取代在S101在先前的研究中产生并研究在机理[元,H.,和耶达,G。(2011)生物化学50,770-779]。在这项研究中,在HEPES,TES或磷酸钠中观察到S101C酶的典型黄素氧化黄酮的典型紫外-可见(UV-vis)吸收光谱,而吸收光谱表明存在半胱氨酸的C4a-黄素加合物。在pH 8.0的Tris-HCl中获得。在存在和不存在Tris的条件下,通过野生型,S101A,S101C和H99N酶的UV-vis吸收光谱的pH滴定,可以测定两个K a值定义了稳定C4a-S-半胱氨酰黄素的pH范围。抑制研究和停流动力学表明,质子化Tris在S101C酶活性位点的结合是形成C4a-S-半胱氨酰黄素所必需的。氘的动力学同位素效应和在Tris的停流分光光度计中混合的S101C酶的质子清单建立了C4a-S-半胱氨酰黄素可逆形成的机制。本研究提供了可逆形成一个bicovalent的C4A-S-半胱8α-N的详细机理分析3 -组氨酰黄素的胆碱氧化酶,识别最优pH范围和用于稳定一个机械原理从头C4a-S-半胱氨酰黄素。此外,其提供了没有底物的酶结合黄素的分子内反应的实例。
更新日期:2017-12-14
中文翻译:

可逆的,电荷诱导的胆碱氧化酶变体S101C中的分子内C4a-S-半胱氨酰-黄素
胆碱氧化酶用作黄素依赖性酶催化的醇氧化的范例。在其活性位点,S101在延伸的环上与黄素C4a原子相距4Å。酶变体取代在S101在先前的研究中产生并研究在机理[元,H.,和耶达,G。(2011)生物化学50,770-779]。在这项研究中,在HEPES,TES或磷酸钠中观察到S101C酶的典型黄素氧化黄酮的典型紫外-可见(UV-vis)吸收光谱,而吸收光谱表明存在半胱氨酸的C4a-黄素加合物。在pH 8.0的Tris-HCl中获得。在存在和不存在Tris的条件下,通过野生型,S101A,S101C和H99N酶的UV-vis吸收光谱的pH滴定,可以测定两个K a值定义了稳定C4a-S-半胱氨酰黄素的pH范围。抑制研究和停流动力学表明,质子化Tris在S101C酶活性位点的结合是形成C4a-S-半胱氨酰黄素所必需的。氘的动力学同位素效应和在Tris的停流分光光度计中混合的S101C酶的质子清单建立了C4a-S-半胱氨酰黄素可逆形成的机制。本研究提供了可逆形成一个bicovalent的C4A-S-半胱8α-N的详细机理分析3 -组氨酰黄素的胆碱氧化酶,识别最优pH范围和用于稳定一个机械原理从头C4a-S-半胱氨酰黄素。此外,其提供了没有底物的酶结合黄素的分子内反应的实例。









































 京公网安备 11010802027423号
京公网安备 11010802027423号