当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Molecular Mechanism of Substrate Recognition and Catalysis of the Membrane Acyltransferase PatA from Mycobacteria
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1021/acschembio.7b00578 Montse Tersa 1 , Lluís Raich 2 , David Albesa-Jové 1, 3, 4, 5 , Beatriz Trastoy 1 , Jacques Prandi 6 , Martine Gilleron 6 , Carme Rovira 2, 7 , Marcelo E. Guerin 1, 3, 4, 5
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1021/acschembio.7b00578 Montse Tersa 1 , Lluís Raich 2 , David Albesa-Jové 1, 3, 4, 5 , Beatriz Trastoy 1 , Jacques Prandi 6 , Martine Gilleron 6 , Carme Rovira 2, 7 , Marcelo E. Guerin 1, 3, 4, 5
Affiliation
|
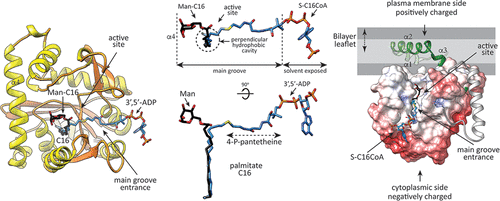
Glycolipids play a central role in a variety of important biological processes in all living organisms. PatA is a membrane acyltransferase involved in the biosynthesis of phosphatidyl-myo-inositol mannosides (PIMs), key structural elements, and virulence factors of Mycobacterium tuberculosis. PatA catalyzes the transfer of a palmitoyl moiety from palmitoyl-CoA to the 6-position of the mannose ring linked to the 2-position of inositol in PIM1/PIM2. We report here the crystal structure of PatA in the presence of 6-O-palmitoyl-α-d-mannopyranoside, unraveling the acceptor binding mechanism. The acceptor mannose ring localizes in a cavity at the end of a surface-exposed long groove where the active site is located, whereas the palmitate moiety accommodates into a hydrophobic pocket deeply buried in the α/β core of the protein. Both fatty acyl chains of the PIM2 acceptor are essential for the reaction to take place, highlighting their critical role in the generation of a competent active site. By the use of combined structural and quantum-mechanics/molecular-mechanics (QM/MM) metadynamics, we unravel the catalytic mechanism of PatA at the atomic-electronic level. Our study provides a detailed structural rationale for a stepwise reaction, with the generation of a tetrahedral transition state for the rate-determining step. Finally, the crystal structure of PatA in the presence of β-d-mannopyranose and palmitate suggests an inhibitory mechanism for the enzyme, providing exciting possibilities for inhibitor design and the discovery of chemotherapeutic agents against this major human pathogen.
中文翻译:

分枝杆菌的膜酰基转移酶PatA的底物识别和催化的分子机理
糖脂在所有活生物体的各种重要生物过程中均起着核心作用。PATA是参与磷脂酰的生物合成的膜酰基转移酶肌肌醇甘露糖苷(PIMS),关键的结构元件,和毒力因子的结核分枝杆菌。PatA催化从Palmitoyl-CoA棕榈酸酯基部分转移到甘露糖环的6位,该位置连接到PIM 1 / PIM 2中肌醇的2位。我们在这里报告在6 - O-棕榈酰-α - d存在下PatA的晶体结构-甘露吡喃糖苷,阐明受体结合机制。受体甘露糖环位于活性部位位于表面暴露的长沟末端的腔中,而棕榈酸酯部分则容纳在深埋在蛋白质α/β核心中的疏水口袋中。PIM 2的两条脂肪酰基链受体对于发生反应是必不可少的,突出了它们在产生有效活性位点中的关键作用。通过使用组合的结构和量子力学/分子力学(QM / MM)的元动力学,我们在原子电子水平上揭示了PatA的催化机理。我们的研究为逐步反应提供了详细的结构原理,并为速率确定步骤生成了四面体过渡态。最后,在β- d-甘露吡喃糖和棕榈酸酯存在下PatA的晶体结构表明了该酶的抑制机制,为抑制剂设计和发现针对这种主要人类病原体的化学治疗剂提供了令人兴奋的可能性。
更新日期:2017-12-11
中文翻译:

分枝杆菌的膜酰基转移酶PatA的底物识别和催化的分子机理
糖脂在所有活生物体的各种重要生物过程中均起着核心作用。PATA是参与磷脂酰的生物合成的膜酰基转移酶肌肌醇甘露糖苷(PIMS),关键的结构元件,和毒力因子的结核分枝杆菌。PatA催化从Palmitoyl-CoA棕榈酸酯基部分转移到甘露糖环的6位,该位置连接到PIM 1 / PIM 2中肌醇的2位。我们在这里报告在6 - O-棕榈酰-α - d存在下PatA的晶体结构-甘露吡喃糖苷,阐明受体结合机制。受体甘露糖环位于活性部位位于表面暴露的长沟末端的腔中,而棕榈酸酯部分则容纳在深埋在蛋白质α/β核心中的疏水口袋中。PIM 2的两条脂肪酰基链受体对于发生反应是必不可少的,突出了它们在产生有效活性位点中的关键作用。通过使用组合的结构和量子力学/分子力学(QM / MM)的元动力学,我们在原子电子水平上揭示了PatA的催化机理。我们的研究为逐步反应提供了详细的结构原理,并为速率确定步骤生成了四面体过渡态。最后,在β- d-甘露吡喃糖和棕榈酸酯存在下PatA的晶体结构表明了该酶的抑制机制,为抑制剂设计和发现针对这种主要人类病原体的化学治疗剂提供了令人兴奋的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号