当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The role of anions in adsorbate-induced anchoring transitions of liquid crystals on surfaces with discrete cation binding sites†
Soft Matter ( IF 2.9 ) Pub Date : 2017-11-29 00:00:00 , DOI: 10.1039/c7sm01981e Tibor Szilvási 1, 2, 3, 4 , Nanqi Bao 1, 2, 3, 4 , Huaizhe Yu 1, 2, 3, 4 , Robert J. Twieg 4, 5, 6, 7 , Manos Mavrikakis 1, 2, 3, 4 , Nicholas L. Abbott 1, 2, 3, 4
Soft Matter ( IF 2.9 ) Pub Date : 2017-11-29 00:00:00 , DOI: 10.1039/c7sm01981e Tibor Szilvási 1, 2, 3, 4 , Nanqi Bao 1, 2, 3, 4 , Huaizhe Yu 1, 2, 3, 4 , Robert J. Twieg 4, 5, 6, 7 , Manos Mavrikakis 1, 2, 3, 4 , Nicholas L. Abbott 1, 2, 3, 4
Affiliation
|
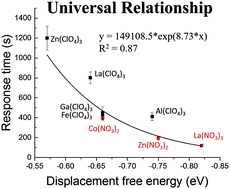
We report a combined theoretical and experimental effort to elucidate systematically for the first time the influence of anions of transition metal salt-decorated surfaces on the orientations of supported films of nematic liquid crystals (LCs) and adsorbate-induced orientational transitions of these LC films. Guided by computational chemistry predictions, we find that nitrate anions weaken the binding of 4′-n-pentyl-4-biphenylcarbonitrile (5CB) to transition metal cations, as compared to perchlorate salts, although binding is still sufficiently strong to induce homeotropic (perpendicular) orientations of 5CB. In addition, we find the orientations of the LC to be correlated across all metal cations investigated by a molecular anchoring energy density that is calculated as the product of the single-site binding energy and metal cation binding site density on the surface. The weaker single-site binding energy caused by nitrate also facilitates competitive binding of adsorbates to the metal cations, leading to more facile orientational transitions induced by adsorbates. Finally, our analysis suggests that nitrate anions recruit water via hydrogen bonding to the metal binding sites, modulating further the relative net binding energies of 5CB and adsorbates to surfaces decorated with metal nitrates. After accounting for the presence of water, we find a universal exponential relationship between the calculated displacement free energies and measured dynamic response of LCs to adsorbates for all metal salts studied, independent of the metal salt anion.
中文翻译:

阴离子在具有离散阳离子结合位点的表面上液晶的吸附物诱导的锚固转变中的作用†
我们报告了结合的理论和实验成果,以系统地首次阐明过渡金属盐修饰的表面的阴离子对向列液晶(LCs)的支撑膜的取向以及这些LC膜的吸附物诱导的取向转变的影响。在计算化学预测的指导下,我们发现硝酸根阴离子会削弱4'- n的结合与高氯酸盐相比,β-戊基-4-联苯甲腈(5CB)过渡金属阳离子,尽管结合仍然足够强,可以诱导5CB的垂直(垂直)取向。此外,我们发现通过分子锚定能量密度调查的所有金属阳离子在LC的方向上是相关的,该分子锚定能量密度计算为表面上单点结合能与金属阳离子结合位点密度的乘积。由硝酸盐引起的较弱的单点结合能也促进了吸附物与金属阳离子的竞争性结合,从而导致由吸附物诱导的更容易的取向转变。最后,我们的分析表明硝酸根阴离子可通过氢键结合到金属结合位点上,进一步调节5CB的相对净结合能,并吸附到装饰有金属硝酸盐的表面上。考虑到水的存在后,我们发现所计算的所有金属盐的计算位移自由能与LCs对吸附物的动态响应之间的通用指数关系,与金属盐阴离子无关。
更新日期:2017-11-29
中文翻译:

阴离子在具有离散阳离子结合位点的表面上液晶的吸附物诱导的锚固转变中的作用†
我们报告了结合的理论和实验成果,以系统地首次阐明过渡金属盐修饰的表面的阴离子对向列液晶(LCs)的支撑膜的取向以及这些LC膜的吸附物诱导的取向转变的影响。在计算化学预测的指导下,我们发现硝酸根阴离子会削弱4'- n的结合与高氯酸盐相比,β-戊基-4-联苯甲腈(5CB)过渡金属阳离子,尽管结合仍然足够强,可以诱导5CB的垂直(垂直)取向。此外,我们发现通过分子锚定能量密度调查的所有金属阳离子在LC的方向上是相关的,该分子锚定能量密度计算为表面上单点结合能与金属阳离子结合位点密度的乘积。由硝酸盐引起的较弱的单点结合能也促进了吸附物与金属阳离子的竞争性结合,从而导致由吸附物诱导的更容易的取向转变。最后,我们的分析表明硝酸根阴离子可通过氢键结合到金属结合位点上,进一步调节5CB的相对净结合能,并吸附到装饰有金属硝酸盐的表面上。考虑到水的存在后,我们发现所计算的所有金属盐的计算位移自由能与LCs对吸附物的动态响应之间的通用指数关系,与金属盐阴离子无关。









































 京公网安备 11010802027423号
京公网安备 11010802027423号