当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of the Marine Alkaloid Discoipyrrole C via the MoOPH-Mediated Oxidation of a 2,3,5-Trisubstituted Pyrrole
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00872 Qiao Yan 1 , Xiang Ma 1 , Martin G. Banwell 1 , Jas S. Ward 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00872 Qiao Yan 1 , Xiang Ma 1 , Martin G. Banwell 1 , Jas S. Ward 1
Affiliation
|
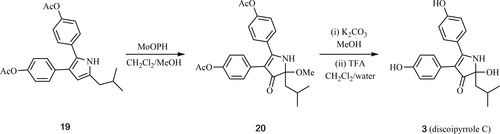
A total synthesis of the marine alkaloid discoipyrrole C (3) is described. In the pivotal step, the 2,3,5-trisubstituted pyrrole 19 was treated with MoOPH in the presence of MeOH, and the resulting methoxylated 1,2-dihydro-3H-pyrrol-3-one 20 subjected to reaction with potassium carbonate in MeOH then trifluoroacetic acid and H2O. This gave a mixture of target 3 and its dehydration product, and the structure of the former compound was confirmed by single-crystal X-ray analysis.
中文翻译:

通过MoOPH介导的2,3,5-三取代的吡咯的氧化反应,全过程合成海洋生物碱DiscoipyrroleC。
描述了海洋生物碱盘二吡咯C(3)的全合成。在关键步骤中,在MeOH的存在下用MoOPH处理2,3,5-三取代的吡咯19,并使所得的甲氧基化的1,2-二氢-3 H-吡咯-3-酮20与碳酸钾反应在MeOH中然后在三氟乙酸和H 2 O中洗脱。这得到目标3及其脱水产物的混合物,并且通过单晶X射线分析确认了前一种化合物的结构。
更新日期:2017-11-28
中文翻译:

通过MoOPH介导的2,3,5-三取代的吡咯的氧化反应,全过程合成海洋生物碱DiscoipyrroleC。
描述了海洋生物碱盘二吡咯C(3)的全合成。在关键步骤中,在MeOH的存在下用MoOPH处理2,3,5-三取代的吡咯19,并使所得的甲氧基化的1,2-二氢-3 H-吡咯-3-酮20与碳酸钾反应在MeOH中然后在三氟乙酸和H 2 O中洗脱。这得到目标3及其脱水产物的混合物,并且通过单晶X射线分析确认了前一种化合物的结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号