当前位置:
X-MOL 学术
›
Environ. Sci. Technol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH Dependence of the Imidazole-2-carboxaldehyde Hydration Equilibrium: Implications for Atmospheric Light Absorbance
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acs.estlett.7b00486 Jessica M. Ackendorf 1 , Michael G. Ippolito 1 , Melissa M. Galloway 1
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acs.estlett.7b00486 Jessica M. Ackendorf 1 , Michael G. Ippolito 1 , Melissa M. Galloway 1
Affiliation
|
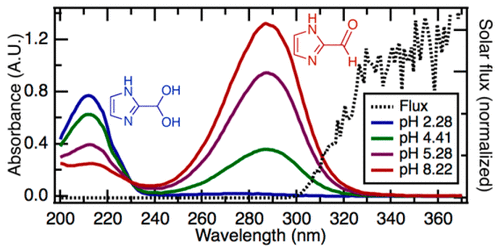
Imidazole-2-carboxaldehyde (IC) has been identified as an aqueous “brown carbon” absorber and a possible atmospheric photosensitizer. IC exists in a pH-dependent equilibrium between its aldehyde and geminal diol form; the diol form is the dominant species in solution at pH <5. Calculated molar absorptivity coefficients are 13700 ± 200 cm–1 M–1 at 287 nm for the aldehyde and 7800 ± 100 cm–1 M–1 at 212 nm for the diol. This shift from aldehyde to diol changes the peak light absorption of the aqueous solution from 287 to 212 nm, which is beyond the actinic range and may have implications for radiative forcing. The observed pH-dependent shift in the hydration equilibrium of IC is driven by the interaction between its hydration and protonation equilibria. Calculated pKa values are 2.5 ± 0.4 and 5.94 ± 0.05 for the aldehyde and diol, respectively, and are consistent with the trend toward increasing diol as the pH decreases. The acid–base equilibrium affects both the solubility and the major species in solution depending on the protonation state of IC and may affect atmospheric light absorption, brown carbon, and photosensitization under acidic conditions. These findings indicate a need for a greater level of attention to the effect of the matrix in aqueous atmospheric systems, particularly concerning species affected by multiple equilibria.
中文翻译:

咪唑-2-甲醛水合平衡的pH依赖性:对大气光吸收的影响
咪唑-2-甲醛(IC)被确定为水性“棕碳”吸收剂和可能的大气光敏剂。IC在其醛和双峰二醇形式之间存在pH依赖性平衡;在pH <5时,二醇形式是溶液中的主要物质。计算出的醛的摩尔吸光系数为287 nm处的13700±200 cm –1 M –1和7800±100 cm –1 M –1二醇在212 nm处 从醛到二醇的这种转变将水溶液的峰值光吸收从287 nm更改为212 nm,这超出了光化范围,可能对辐射强迫有影响。观察到的IC的水合平衡中pH依赖的变化是由IC的水合与质子化平衡之间的相互作用驱动的。计算出的p K a对于醛和二醇,该值分别为2.5±0.4和5.94±0.05,并且与随着pH降低而使二醇增加的趋势一致。酸碱平衡会影响溶解度和溶液中的主要物质,这取决于IC的质子化状态,并且可能会影响酸性条件下的大气光吸收,褐碳和光敏性。这些发现表明需要更多地关注基质在含水大气系统中的作用,特别是涉及受多重平衡影响的物种。
更新日期:2017-11-29
中文翻译:

咪唑-2-甲醛水合平衡的pH依赖性:对大气光吸收的影响
咪唑-2-甲醛(IC)被确定为水性“棕碳”吸收剂和可能的大气光敏剂。IC在其醛和双峰二醇形式之间存在pH依赖性平衡;在pH <5时,二醇形式是溶液中的主要物质。计算出的醛的摩尔吸光系数为287 nm处的13700±200 cm –1 M –1和7800±100 cm –1 M –1二醇在212 nm处 从醛到二醇的这种转变将水溶液的峰值光吸收从287 nm更改为212 nm,这超出了光化范围,可能对辐射强迫有影响。观察到的IC的水合平衡中pH依赖的变化是由IC的水合与质子化平衡之间的相互作用驱动的。计算出的p K a对于醛和二醇,该值分别为2.5±0.4和5.94±0.05,并且与随着pH降低而使二醇增加的趋势一致。酸碱平衡会影响溶解度和溶液中的主要物质,这取决于IC的质子化状态,并且可能会影响酸性条件下的大气光吸收,褐碳和光敏性。这些发现表明需要更多地关注基质在含水大气系统中的作用,特别是涉及受多重平衡影响的物种。









































 京公网安备 11010802027423号
京公网安备 11010802027423号