当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bimetallic Carbide as a Stable Hydrogen Evolution Catalyst in Harsh Acidic Water
ACS Energy Letters ( IF 19.3 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acsenergylett.7b00990 Meng Yang Zu 1 , Peng Fei Liu 1 , Chongwu Wang 1 , Yun Wang 2 , Li Rong Zheng 3 , Bo Zhang 4 , Huijun Zhao 2 , Hua Gui Yang 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acsenergylett.7b00990 Meng Yang Zu 1 , Peng Fei Liu 1 , Chongwu Wang 1 , Yun Wang 2 , Li Rong Zheng 3 , Bo Zhang 4 , Huijun Zhao 2 , Hua Gui Yang 1
Affiliation
|
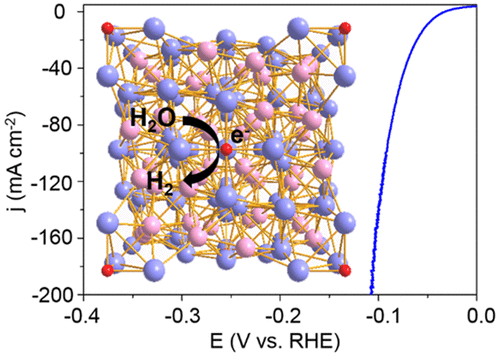
Cheap, efficient, and stable hydrogen evolution reaction (HER) electrocatalysts have long been pursued, owing to their scientific and technological importance. Currently, platinum has been regarded as the benchmarked HER electrocatalyst. Unfortunately, the low abundance and high cost impede its industrial applications. Here, we synthesize bimetallic carbide Mo6Ni6C grown on nickel foam as a HER catalyst, delivering a low overpotential of −51 mV at −10 mA cm–2 in 0.5 M H2SO4 for more than 200 h, which is among the best reported benchmarked HER catalysts in acid to date. On the basis of experimental observations and theoretical modeling, we ascribe the good activity to the proper Gibbs free energy of adsorbed hydrogen (ΔG(H*)) for the carbon active sites and attribute the stability to the corrosion-stable Mo–Mo bonds in the crystal structure. This work demonstrates the possibility for Mo6Ni6C to be one of the best candidates for HER electrocatalysts in the large-scale electrolysis industry.
中文翻译:

双金属碳化物在苛刻酸性水中的稳定产氢催化剂
由于其科学和技术重要性,长期以来一直在寻求廉价,有效和稳定的氢析出反应(HER)电催化剂。当前,铂已经被认为是基准的HER电催化剂。不幸的是,低丰度和高成本阻碍了其工业应用。在这里,我们合成了在镍泡沫上生长的双金属碳化物Mo 6 Ni 6 C作为HER催化剂,在0.5 MH 2 SO 4中在−10 mA cm –2时提供了−51 mV的低过电势超过200小时,这是迄今为止在酸中报道最多的基准HER催化剂之一。根据实验观察和理论建模,我们将良好的活性归因于碳活性位的适当吸附氢的吉布斯自由能(ΔG(H *)),并将稳定性归因于腐蚀稳定的Mo-Mo键在晶体结构中。这项工作证明了Mo 6 Ni 6 C可能成为大规模电解工业中HER电催化剂的最佳候选者之一。
更新日期:2017-11-28
中文翻译:

双金属碳化物在苛刻酸性水中的稳定产氢催化剂
由于其科学和技术重要性,长期以来一直在寻求廉价,有效和稳定的氢析出反应(HER)电催化剂。当前,铂已经被认为是基准的HER电催化剂。不幸的是,低丰度和高成本阻碍了其工业应用。在这里,我们合成了在镍泡沫上生长的双金属碳化物Mo 6 Ni 6 C作为HER催化剂,在0.5 MH 2 SO 4中在−10 mA cm –2时提供了−51 mV的低过电势超过200小时,这是迄今为止在酸中报道最多的基准HER催化剂之一。根据实验观察和理论建模,我们将良好的活性归因于碳活性位的适当吸附氢的吉布斯自由能(ΔG(H *)),并将稳定性归因于腐蚀稳定的Mo-Mo键在晶体结构中。这项工作证明了Mo 6 Ni 6 C可能成为大规模电解工业中HER电催化剂的最佳候选者之一。











































 京公网安备 11010802027423号
京公网安备 11010802027423号