当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anticancer Properties of Halogenated Pyrrolo[3,2‐d]pyrimidines with Decreased Toxicity via N5 Substitution
ChemMedChem ( IF 3.6 ) Pub Date : 2017-12-18 , DOI: 10.1002/cmdc.201700641 Brian M Cawrse 1 , Rena S Lapidus 2 , Brandon Cooper 2 , Eun Yong Choi 2 , Katherine L Seley-Radtke 1
ChemMedChem ( IF 3.6 ) Pub Date : 2017-12-18 , DOI: 10.1002/cmdc.201700641 Brian M Cawrse 1 , Rena S Lapidus 2 , Brandon Cooper 2 , Eun Yong Choi 2 , Katherine L Seley-Radtke 1
Affiliation
|
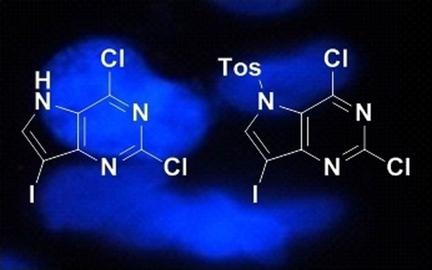
Halogenated pyrrolo[3,2‐d]pyrimidine analogues have shown antiproliferative activity in recent studies, with cell accumulation occurring in the G2/M stage without apoptosis. However, the mechanism of action and pharmacokinetic (PK) profile of these compounds has yet to be determined. To investigate the PK profile of these compounds, a series of halogenated pyrrolo[3,2‐d]pyrimidine compounds was synthesized and first tested for activity in various cancer cell lines followed by a mouse model. EC50 values ranged from 0.014 to 14.5 μm, and maximum tolerated doses (MTD) in mice were between 5 and 10 mg kg−1. This indicates a wide variance in activity and toxicity that necessitates further study. To decrease toxicity, a second series of compounds was synthesized with N5‐alkyl substitutions in an effort to slow the rate of metabolism, which was thought to be leading to the toxicity. The N‐substituted compounds demonstrated comparable cell line activity (EC50 values between 0.83–7.3 μm) with significantly decreased toxicity (MTD=40 mg kg−1). Finally, the PK profile of the active N5‐substituted compound shows a plasma half‐life of 32.7 minutes, and rapid conversion into the parent unsubstituted analogue. Together, these data indicate that halogenated pyrrolo[3,2‐d]pyrimidines present a promising lead into potent antiproliferative agents with tunable activity and toxicity, and rapid metabolism.
中文翻译:

通过 N5 取代降低毒性的卤代吡咯并[3,2-d]嘧啶的抗癌特性
卤代吡咯并[3,2- d ]嘧啶类似物在最近的研究中显示出抗增殖活性,细胞积聚发生在G 2 /M阶段而没有凋亡。然而,这些化合物的作用机制和药代动力学 (PK) 特征尚未确定。为了研究这些化合物的 PK 特性,合成了一系列卤代吡咯并[3,2- d ]嘧啶化合物,并首先测试了在各种癌细胞系中的活性,然后在小鼠模型中进行了测试。EC 50值范围为0.014至14.5μm ,小鼠最大耐受剂量(MTD)为5至10mg kg -1。这表明活性和毒性存在很大差异,需要进一步研究。为了降低毒性,合成了带有 N5-烷基取代基的第二系列化合物,以减缓代谢速率,这被认为是导致毒性的原因。N取代的化合物表现出可比的细胞系活性(EC 50值在0.83–7.3 μ m之间),并且毒性显着降低(MTD=40 mg kg -1)。最后,活性 N5 取代化合物的 PK 曲线显示血浆半衰期为 32.7 分钟,并快速转化为母体未取代类似物。总之,这些数据表明,卤代吡咯并[3,2- d ]嘧啶有望成为有效的抗增殖剂,具有可调节的活性和毒性以及快速代谢。
更新日期:2017-12-18
中文翻译:

通过 N5 取代降低毒性的卤代吡咯并[3,2-d]嘧啶的抗癌特性
卤代吡咯并[3,2- d ]嘧啶类似物在最近的研究中显示出抗增殖活性,细胞积聚发生在G 2 /M阶段而没有凋亡。然而,这些化合物的作用机制和药代动力学 (PK) 特征尚未确定。为了研究这些化合物的 PK 特性,合成了一系列卤代吡咯并[3,2- d ]嘧啶化合物,并首先测试了在各种癌细胞系中的活性,然后在小鼠模型中进行了测试。EC 50值范围为0.014至14.5μm ,小鼠最大耐受剂量(MTD)为5至10mg kg -1。这表明活性和毒性存在很大差异,需要进一步研究。为了降低毒性,合成了带有 N5-烷基取代基的第二系列化合物,以减缓代谢速率,这被认为是导致毒性的原因。N取代的化合物表现出可比的细胞系活性(EC 50值在0.83–7.3 μ m之间),并且毒性显着降低(MTD=40 mg kg -1)。最后,活性 N5 取代化合物的 PK 曲线显示血浆半衰期为 32.7 分钟,并快速转化为母体未取代类似物。总之,这些数据表明,卤代吡咯并[3,2- d ]嘧啶有望成为有效的抗增殖剂,具有可调节的活性和毒性以及快速代谢。











































 京公网安备 11010802027423号
京公网安备 11010802027423号