当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid-State Hydrogen–Deuterium Exchange Mass Spectrometry: Correlation of Deuterium Uptake and Long-Term Stability of Lyophilized Monoclonal Antibody Formulations
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00504 Balakrishnan S. Moorthy 1 , Isidro E. Zarraga 2 , Lokesh Kumar 3 , Benjamin T. Walters 4 , Pierre Goldbach 5 , Elizabeth M. Topp 1 , Andrea Allmendinger 5
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00504 Balakrishnan S. Moorthy 1 , Isidro E. Zarraga 2 , Lokesh Kumar 3 , Benjamin T. Walters 4 , Pierre Goldbach 5 , Elizabeth M. Topp 1 , Andrea Allmendinger 5
Affiliation
|
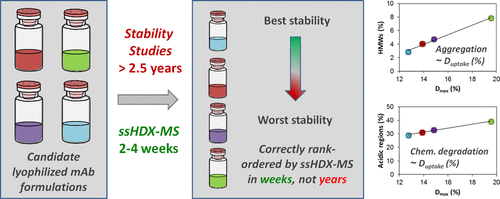
Solid state hydrogen–deuterium exchange with mass spectrometric analysis (ssHDX-MS) has been used to assess protein conformation and matrix interactions in lyophilized solids. ssHDX-MS metrics have been previously correlated to the formation of aggregates of lyophilized myoglobin on storage. Here, ssHDX-MS was applied to lyophilized monoclonal antibody (mAb) formulations and correlated to their long-term stability. After exposing lyophilized samples to D2O(g), the amount of deuterium incorporated at various time points was determined by mass spectrometry for four different lyophilized mAb formulations. Hydrogen–deuterium exchange data were then correlated with mAb aggregation and chemical degradation, which was obtained in stability studies of >2.5 years. Deuterium uptake on ssHDX-MS of four lyophilized mAb formulations determined at the initial time point prior to storage in the dry state was directly and strongly correlated with the extent of aggregation and chemical degradation during storage. Other measures of physical and chemical properties of the solids were weakly or poorly correlated with stability. The data demonstrate, for the first time, that ssHDX-MS results are highly correlated with the stability of lyophilized mAb formulations. The findings thus suggest that ssHDX-MS can be used as an early read-out of differences in long-term stability between formulations helping to accelerate formulation screening and selection.
中文翻译:

固态氢-氘交换质谱:氘摄取与冻干单克隆抗体制剂的长期稳定性的相关性
固态氢-氘交换与质谱分析(ssHDX-MS)已用于评估冻干固体中的蛋白质构象和基质相互作用。ssHDX-MS指标先前已与储存中冻干的肌红蛋白聚集体的形成相关。在这里,ssHDX-MS被应用于冻干的单克隆抗体(mAb)制剂,并与其长期稳定性相关。将冻干的样品暴露于D 2后O(g),通过质谱测定四种不同的冻干mAb制剂在各个时间点掺入的氘的量。然后,氢-氘交换数据与mAb聚集和化学降解相关,这是通过> 2.5年的稳定性研究获得的。在干燥状态下储存之前在初始时间点确定的四种冻干mAb制剂在ssHDX-MS上的氘吸收与储存过程中的聚集程度和化学降解程度密切相关。固体的其他物理和化学性质的度量值与稳定性的相关性较弱或较弱。数据首次证明ssHDX-MS结果与冻干mAb制剂的稳定性高度相关。
更新日期:2017-11-28
中文翻译:

固态氢-氘交换质谱:氘摄取与冻干单克隆抗体制剂的长期稳定性的相关性
固态氢-氘交换与质谱分析(ssHDX-MS)已用于评估冻干固体中的蛋白质构象和基质相互作用。ssHDX-MS指标先前已与储存中冻干的肌红蛋白聚集体的形成相关。在这里,ssHDX-MS被应用于冻干的单克隆抗体(mAb)制剂,并与其长期稳定性相关。将冻干的样品暴露于D 2后O(g),通过质谱测定四种不同的冻干mAb制剂在各个时间点掺入的氘的量。然后,氢-氘交换数据与mAb聚集和化学降解相关,这是通过> 2.5年的稳定性研究获得的。在干燥状态下储存之前在初始时间点确定的四种冻干mAb制剂在ssHDX-MS上的氘吸收与储存过程中的聚集程度和化学降解程度密切相关。固体的其他物理和化学性质的度量值与稳定性的相关性较弱或较弱。数据首次证明ssHDX-MS结果与冻干mAb制剂的稳定性高度相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号