当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inter-Enzyme Allosteric Regulation of Chorismate Mutase in Corynebacterium glutamicum: Structural Basis of Feedback Activation by Trp
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1021/acs.biochem.7b01018 Daniel Burschowsky 1 , Helen V. Thorbjørnsrud 1 , Joel B. Heim 1 , Ju̅ratė Fahrig-Kamarauskaitė 2 , Kathrin Würth-Roderer 2 , Peter Kast 2 , Ute Krengel 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1021/acs.biochem.7b01018 Daniel Burschowsky 1 , Helen V. Thorbjørnsrud 1 , Joel B. Heim 1 , Ju̅ratė Fahrig-Kamarauskaitė 2 , Kathrin Würth-Roderer 2 , Peter Kast 2 , Ute Krengel 1
Affiliation
|
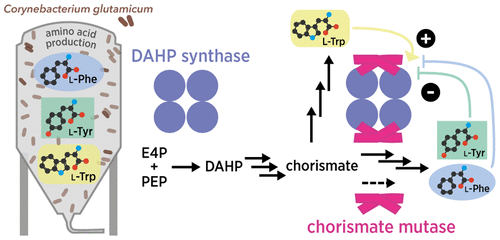
Corynebacterium glutamicum is widely used for the industrial production of amino acids, nucleotides, and vitamins. The shikimate pathway enzymes DAHP synthase (CgDS, Cg2391) and chorismate mutase (CgCM, Cgl0853) play a key role in the biosynthesis of aromatic compounds. Here we show that CgCM requires the formation of a complex with CgDS to achieve full activity, and that both CgCM and CgDS are feedback regulated by aromatic amino acids binding to CgDS. Kinetic analysis showed that Phe and Tyr inhibit CgCM activity by inter-enzyme allostery, whereas binding of Trp to CgDS strongly activates CgCM. Mechanistic insights were gained from crystal structures of the CgCM homodimer, tetrameric CgDS, and the heterooctameric CgCM–CgDS complex, refined to 1.1, 2.5, and 2.2 Å resolution, respectively. Structural details from the allosteric binding sites reveal that DAHP synthase is recruited as the dominant regulatory platform to control the shikimate pathway, similar to the corresponding enzyme complex from Mycobacterium tuberculosis.
中文翻译:

谷氨酸棒状杆菌分支酸突变酶的酶间变构调节:Trp反馈激活的结构基础
谷氨酸棒杆菌被广泛用于氨基酸,核苷酸和维生素的工业生产。sh草酸途径酶DAHP合酶(CgDS,Cg2391)和分支酸突变酶(CgCM,Cgl0853)在芳香族化合物的生物合成中起关键作用。在这里,我们显示CgCM需要与CgDS形成复合物才能实现完全活性,并且CgCM和CgDS都受到与CgDS结合的芳香族氨基酸的反馈调控。动力学分析表明,Phe和Tyr通过酶间变构抑制CgCM活性,而Trp与CgDS的结合强烈激活CgCM。从CgCM同型二聚体,四聚体CgDS和异八聚体CgCM–CgDS复合物的晶体结构中获得了机理方面的见解,分别将其精炼为1.1、2.5和2.2Å的分辨率。结核分枝杆菌。
更新日期:2017-12-21
中文翻译:

谷氨酸棒状杆菌分支酸突变酶的酶间变构调节:Trp反馈激活的结构基础
谷氨酸棒杆菌被广泛用于氨基酸,核苷酸和维生素的工业生产。sh草酸途径酶DAHP合酶(CgDS,Cg2391)和分支酸突变酶(CgCM,Cgl0853)在芳香族化合物的生物合成中起关键作用。在这里,我们显示CgCM需要与CgDS形成复合物才能实现完全活性,并且CgCM和CgDS都受到与CgDS结合的芳香族氨基酸的反馈调控。动力学分析表明,Phe和Tyr通过酶间变构抑制CgCM活性,而Trp与CgDS的结合强烈激活CgCM。从CgCM同型二聚体,四聚体CgDS和异八聚体CgCM–CgDS复合物的晶体结构中获得了机理方面的见解,分别将其精炼为1.1、2.5和2.2Å的分辨率。结核分枝杆菌。











































 京公网安备 11010802027423号
京公网安备 11010802027423号