当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of the Stereoselective Synthesis of the Indane Dimer PH46A, a New Potential Anti-inflammatory Agent.
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-11-27 , DOI: 10.1021/acs.oprd.7b00258 Graham R Cumming 1 , Tao Zhang 2 , Gaia Scalabrino 2 , Neil Frankish 2, 3 , Helen Sheridan 2, 3
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-11-27 , DOI: 10.1021/acs.oprd.7b00258 Graham R Cumming 1 , Tao Zhang 2 , Gaia Scalabrino 2 , Neil Frankish 2, 3 , Helen Sheridan 2, 3
Affiliation
|
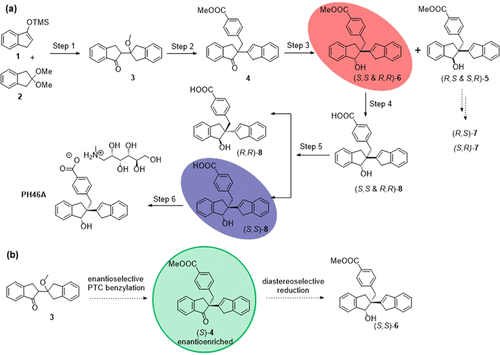
PH46A, belonging to a class of 1,2-Indane dimers, has been developed by our research group as a potential therapeutic agent for the treatment of inflammatory and autoimmune diseases. The initial synthetic route to PH46A gave a low overall yield, due in large part to the generation of undesired diastereoisomer 5 and the unwanted enantiomer (R,R)-8 during the synthesis. The aim of this work was to carry out a comprehensive investigation into the stereoselective synthesis of PH46A. Significant progress was made on the ketone reduction step, where the use of triisobutylaluminum [TiBA, Al(iBu)3] afforded high selectivity for the target diastereoisomer (rac)-6, compared to the unfavorable ratio obtained using a previous process. This enabled a multikilo scale synthesis of PH46A in a GMP environment. Further, a brief proof-of-principle investigation was carried out using an achiral phase transfer catalyst (PTC) for alkylation at the methine carbon of the parent indanone.
中文翻译:

新型潜在抗炎药茚满二聚体PH46A的立体选择性合成研究。
PH46A属于1,2-茚满二聚体,是我们的研究小组开发的,可作为治疗炎症和自身免疫性疾病的潜在治疗剂。最初合成PH46A的途径总产率较低,这在很大程度上是由于合成过程中产生了非所需的非对映异构体5和不需要的对映异构体(R,R)-8。这项工作的目的是对PH46A的立体选择性合成进行全面的研究。酮还原步骤取得了重大进展,与使用先前方法获得的不利比例相比,使用三异丁基铝[TiBA,Al(iBu)3]对目标非对映异构体(rac)-6的选择性高。这使得能够在GMP环境中进行PH46A的多千克级合成。进一步,
更新日期:2017-12-06
中文翻译:

新型潜在抗炎药茚满二聚体PH46A的立体选择性合成研究。
PH46A属于1,2-茚满二聚体,是我们的研究小组开发的,可作为治疗炎症和自身免疫性疾病的潜在治疗剂。最初合成PH46A的途径总产率较低,这在很大程度上是由于合成过程中产生了非所需的非对映异构体5和不需要的对映异构体(R,R)-8。这项工作的目的是对PH46A的立体选择性合成进行全面的研究。酮还原步骤取得了重大进展,与使用先前方法获得的不利比例相比,使用三异丁基铝[TiBA,Al(iBu)3]对目标非对映异构体(rac)-6的选择性高。这使得能够在GMP环境中进行PH46A的多千克级合成。进一步,











































 京公网安备 11010802027423号
京公网安备 11010802027423号