当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Long-Range Changes in Neurolysin Dynamics Upon Inhibitor Binding
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2017-12-08 00:00:00 , DOI: 10.1021/acs.jctc.7b00944 A. Uyar 1 , V. T. Karamyan 2 , A. Dickson 1, 3
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2017-12-08 00:00:00 , DOI: 10.1021/acs.jctc.7b00944 A. Uyar 1 , V. T. Karamyan 2 , A. Dickson 1, 3
Affiliation
|
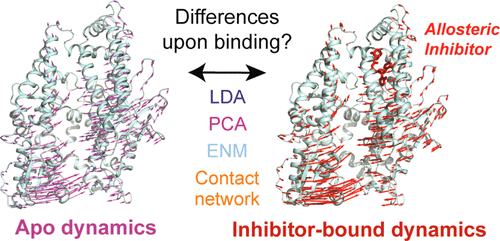
Crystal structures of neurolysin, a zinc metallopeptidase, do not show a significant conformational change upon the binding of an allosteric inhibitor. Neurolysin has a deep channel where it hydrolyzes a short neuropeptide neurotensin to create inactive fragments and thus controls its level in the tissue. Neurolysin is of interest as a therapeutic target since changes in neurotensin level have been implicated in cardiovascular disorders, neurological disorders, and cancer, and inhibitors of neurolysin have been developed. An understanding of the dynamical and structural differences between apo and inhibitor-bound neurolysin will aid in further design of potent inhibitors and activators. For this purpose, we performed several molecular dynamics (MD) simulations for both apo and inhibitor-bound neurolysin. A machine learning method (Linear Discriminant Analysis) is applied to reveal differences between the apo and inhibitor-bound ensembles in an automated way, and large differences are observed on residues that are far from both the active site and the inhibitor binding site. The effects of inhibitor binding on the collective motions of neurolysin are extensively analyzed and compared using both Principal Component Analysis and Elastic Network Model calculations. We find that inhibitor binding induces additional low-frequency motions that are not observed in the apo form. ENM also reveals changes in inter- and intradomain communication upon binding. Furthermore, differences are observed in the inhibitor-bound neurolysin contact network that are far from the active site, revealing long-range allosteric behavior. This study also provides insight into the allosteric modulation of other neuropeptidases with similar folds.
中文翻译:

抑制剂结合后神经溶素动力学的远距离变化
神经溶素(一种锌金属肽酶)的晶体结构在变构抑制剂结合后没有显示出明显的构象变化。神经溶素具有深通道,可在其中水解短的神经肽神经降压素以产生失活的片段,从而控制其在组织中的水平。神经溶解素作为治疗靶标是令人感兴趣的,因为神经降压素水平的改变与心血管疾病,神经系统疾病和癌症有关,并且已经开发了神经溶解素的抑制剂。了解载脂蛋白和结合抑制剂的神经溶素之间的动力学和结构差异将有助于进一步设计有效的抑制剂和活化剂。为此,我们对载脂蛋白和抑制剂结合的神经溶素进行了几种分子动力学(MD)模拟。应用机器学习方法(线性判别分析)以自动方式揭示载脂蛋白和抑制剂结合的集合之间的差异,并且在远离活性位点和抑制剂结合位点的残基上观察到很大的差异。使用主成分分析和弹性网络模型计算对抑制剂结合对神经溶素集体运动的影响进行了广泛的分析和比较。我们发现抑制剂结合诱导了载脂蛋白形式中未观察到的其他低频运动。ENM还揭示了绑定后域间和域内通信的变化。此外,在与抑制剂结合的神经溶素接触网络中观察到与活性部位相距甚远的差异,从而揭示了远距离的变构行为。
更新日期:2017-12-08
中文翻译:

抑制剂结合后神经溶素动力学的远距离变化
神经溶素(一种锌金属肽酶)的晶体结构在变构抑制剂结合后没有显示出明显的构象变化。神经溶素具有深通道,可在其中水解短的神经肽神经降压素以产生失活的片段,从而控制其在组织中的水平。神经溶解素作为治疗靶标是令人感兴趣的,因为神经降压素水平的改变与心血管疾病,神经系统疾病和癌症有关,并且已经开发了神经溶解素的抑制剂。了解载脂蛋白和结合抑制剂的神经溶素之间的动力学和结构差异将有助于进一步设计有效的抑制剂和活化剂。为此,我们对载脂蛋白和抑制剂结合的神经溶素进行了几种分子动力学(MD)模拟。应用机器学习方法(线性判别分析)以自动方式揭示载脂蛋白和抑制剂结合的集合之间的差异,并且在远离活性位点和抑制剂结合位点的残基上观察到很大的差异。使用主成分分析和弹性网络模型计算对抑制剂结合对神经溶素集体运动的影响进行了广泛的分析和比较。我们发现抑制剂结合诱导了载脂蛋白形式中未观察到的其他低频运动。ENM还揭示了绑定后域间和域内通信的变化。此外,在与抑制剂结合的神经溶素接触网络中观察到与活性部位相距甚远的差异,从而揭示了远距离的变构行为。



























 京公网安备 11010802027423号
京公网安备 11010802027423号