当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Binding of Polythiophenes to Amyloids: Structural Mapping of the Pharmacophore
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2017-11-27 00:00:00 , DOI: 10.1021/acschemneuro.7b00397 Anne K. Schütz 1 , Simone Hornemann 2 , Marielle A. Wälti 1 , Ladina Greuter 2 , Cinzia Tiberi 2 , Riccardo Cadalbert 1 , Matthias Gantner 1 , Roland Riek 1 , Per Hammarström 3 , K. Peter R. Nilsson 3 , Anja Böckmann 4 , Adriano Aguzzi 2 , Beat H. Meier 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2017-11-27 00:00:00 , DOI: 10.1021/acschemneuro.7b00397 Anne K. Schütz 1 , Simone Hornemann 2 , Marielle A. Wälti 1 , Ladina Greuter 2 , Cinzia Tiberi 2 , Riccardo Cadalbert 1 , Matthias Gantner 1 , Roland Riek 1 , Per Hammarström 3 , K. Peter R. Nilsson 3 , Anja Böckmann 4 , Adriano Aguzzi 2 , Beat H. Meier 1
Affiliation
|
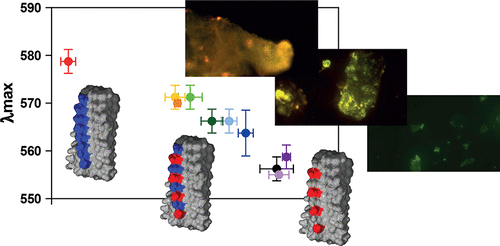
Luminescent conjugated polythiophenes bind to amyloid proteins with high affinity. Their fluorescence properties, which are modulated by the detailed conformation in the bound state, are highly sensitive to structural features of the amyloid. Polythiophenes therefore represent diagnostic markers for the detection and differentiation of pathological amyloid aggregates. We clarify the binding site and mode of two different polythiophenes to fibrils of the prion domain of the HET-s protein by solid-state NMR and correlate these findings with their fluorescence properties. We demonstrate how amyloid dyes recognize distinct binding sites with specific topological features. Regularly spaced surface charge patterns and well-accessible grooves on the fibril surface define the pharmacophore of the amyloid, which in turn determines the binding mode and fluorescence wavelength of the polythiophene.
中文翻译:

聚噻吩与淀粉样蛋白的结合:药效基团的结构图
发光共轭聚噻吩以高亲和力与淀粉样蛋白结合。它们的荧光特性在结合状态下受到详细构象的调节,对淀粉样蛋白的结构特征高度敏感。因此,聚噻吩代表了用于检测和区分病理性淀粉样蛋白聚集体的诊断标记。我们通过固态NMR阐明了两种不同的聚噻吩与HET-s蛋白的pr病毒结构域的原纤维的结合位点和模式,并将这些发现与其荧光性质相关联。我们证明淀粉样蛋白染料如何识别具有特定拓扑特征的独特结合位点。规则间隔的表面电荷模式和原纤维表面上易于接近的凹槽定义了淀粉样蛋白的药效基团,
更新日期:2017-11-27
中文翻译:

聚噻吩与淀粉样蛋白的结合:药效基团的结构图
发光共轭聚噻吩以高亲和力与淀粉样蛋白结合。它们的荧光特性在结合状态下受到详细构象的调节,对淀粉样蛋白的结构特征高度敏感。因此,聚噻吩代表了用于检测和区分病理性淀粉样蛋白聚集体的诊断标记。我们通过固态NMR阐明了两种不同的聚噻吩与HET-s蛋白的pr病毒结构域的原纤维的结合位点和模式,并将这些发现与其荧光性质相关联。我们证明淀粉样蛋白染料如何识别具有特定拓扑特征的独特结合位点。规则间隔的表面电荷模式和原纤维表面上易于接近的凹槽定义了淀粉样蛋白的药效基团,



























 京公网安备 11010802027423号
京公网安备 11010802027423号