当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
“Dressing up” an Old Drug: An Aminoacyl Lipid for the Functionalization of Ru(III)-Based Anticancer Agents
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1021/acsbiomaterials.7b00547 Claudia Riccardi 1 , Domenica Musumeci 1, 2 , Antonella Capuozzo 3 , Carlo Irace 3 , Stephen King 4 , Irene Russo Krauss 1, 5 , Luigi Paduano 1, 5 , Daniela Montesarchio 1, 6
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1021/acsbiomaterials.7b00547 Claudia Riccardi 1 , Domenica Musumeci 1, 2 , Antonella Capuozzo 3 , Carlo Irace 3 , Stephen King 4 , Irene Russo Krauss 1, 5 , Luigi Paduano 1, 5 , Daniela Montesarchio 1, 6
Affiliation
|
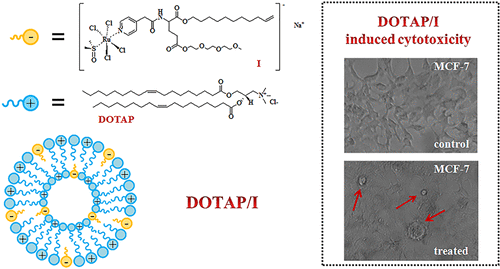
In the search for more efficient anticancer treatments, Ru(III) complexes have attracted much interest among metal-based candidate drugs, showing marked antitumor and antimetastatic activity associated with lower systemic toxicity. Remarkable examples are the Ru(III) complexes NAMI-A and KP1019, which have reached advanced clinical evaluation. In order to improve the in vivo stability of Ru(III)-based drugs, as well as their cellular uptake and effectiveness, a new approach has been proposed by our research group, based on the incorporation of the active, NAMI-A-like Ru(III) complex into highly functionalized nucleolipidic structures, i.e., hybrid molecules containing a nucleoside or nucleotide central core derivatized with a lipid chain, ensuring both efficient protection against extracellular degradation and high cellular internalization of the metal. Aiming at expanding the chemical diversity of available amphiphilic Ru(III) complexes, we here selected a trifunctional α-amino acid to replace the nucleosidic core of previously prepared nucleolipid-based Ru(III) complexes. The amino acidic scaffold, linked to the Ru(III) complex, is decorated with both hydrophilic and lipophilic moieties, conferring high propensity to form stable aggregates in water, which is required to obtain a suitable nanocarrier for the drug delivery. Following this approach, a novel compound, indicated here as compound I, was successfully prepared and characterized, then studied in coformulation with the biocompatible cationic lipid 1,2-dioleyl-3-trimethylammoniumpropane chloride (DOTAP) by dynamic light scattering (DLS), small angle neutron scattering (SANS), and UV–vis analysis. Evaluated in vitro on a panel of human and nonhuman cell lines, it showed good antiproliferative activity on cancer cells, with IC50 values in the μM range, and no relevant cytotoxicity on the healthy cells used as control.
中文翻译:

“修饰”一种旧药:一种基于Ru(III)的抗癌剂功能化的氨酰脂质
在寻求更有效的抗癌治疗中,Ru(III)配合物在基于金属的候选药物中引起了极大的兴趣,显示出与较低的全身毒性相关的显着的抗肿瘤和抗转移活性。杰出的例子是Ru(III)络合物NAMI-A和KP1019,它们已经得到了先进的临床评估。为了提高基于Ru(III)的药物的体内稳定性以及它们的细胞吸收和有效性,我们的研究小组基于掺入活性NAMI-A样的药物,提出了一种新方法。 Ru(III)络合成高度官能化的核脂结构,即含有通过脂质链衍生化的核苷或核苷酸中心核的杂化分子,确保有效地防止金属的细胞外降解和金属的高度细胞内在化。为了扩大可用两亲性Ru(III)配合物的化学多样性,我们在这里选择了三官能团的α-氨基酸来取代先前制备的基于核苷的Ru(III)配合物的核苷核心。与Ru(III)配合物相连的氨基酸支架被亲水性和亲脂性部分修饰,赋予了在水中形成稳定聚集体的高倾向性,这是获得合适的纳米载体进行药物递送所必需的。按照这种方法,一种新的化合物,在这里表示为化合物 我们在这里选择了一个三官能的α-氨基酸来取代先前制备的基于核糖的Ru(III)配合物的核苷核心。与Ru(III)配合物相连的氨基酸支架被亲水性和亲脂性部分修饰,赋予了在水中形成稳定聚集体的高倾向性,这是获得合适的纳米载体进行药物递送所必需的。按照这种方法,一种新的化合物,在这里表示为化合物 我们在这里选择了一个三官能的α-氨基酸来取代先前制备的基于核糖的Ru(III)配合物的核苷核心。与Ru(III)配合物相连的氨基酸支架被亲水性和亲脂性部分修饰,赋予了在水中形成稳定聚集体的高倾向性,这是获得合适的纳米载体进行药物递送所必需的。按照这种方法,一种新的化合物,在这里表示为化合物I是成功制备和表征的,然后通过动态光散射(DLS),小角中子散射(SANS)和UV-vis与生物相容性阳离子脂质1,2-二醇基-3-三甲基铵丙烷氯化物(DOTAP)共同配制。分析。在一组人类和非人类细胞系上进行了体外评估,它显示出对癌细胞良好的抗增殖活性,IC 50值在μM范围内,并且对用作对照的健康细胞没有相关的细胞毒性。
更新日期:2017-12-11
中文翻译:

“修饰”一种旧药:一种基于Ru(III)的抗癌剂功能化的氨酰脂质
在寻求更有效的抗癌治疗中,Ru(III)配合物在基于金属的候选药物中引起了极大的兴趣,显示出与较低的全身毒性相关的显着的抗肿瘤和抗转移活性。杰出的例子是Ru(III)络合物NAMI-A和KP1019,它们已经得到了先进的临床评估。为了提高基于Ru(III)的药物的体内稳定性以及它们的细胞吸收和有效性,我们的研究小组基于掺入活性NAMI-A样的药物,提出了一种新方法。 Ru(III)络合成高度官能化的核脂结构,即含有通过脂质链衍生化的核苷或核苷酸中心核的杂化分子,确保有效地防止金属的细胞外降解和金属的高度细胞内在化。为了扩大可用两亲性Ru(III)配合物的化学多样性,我们在这里选择了三官能团的α-氨基酸来取代先前制备的基于核苷的Ru(III)配合物的核苷核心。与Ru(III)配合物相连的氨基酸支架被亲水性和亲脂性部分修饰,赋予了在水中形成稳定聚集体的高倾向性,这是获得合适的纳米载体进行药物递送所必需的。按照这种方法,一种新的化合物,在这里表示为化合物 我们在这里选择了一个三官能的α-氨基酸来取代先前制备的基于核糖的Ru(III)配合物的核苷核心。与Ru(III)配合物相连的氨基酸支架被亲水性和亲脂性部分修饰,赋予了在水中形成稳定聚集体的高倾向性,这是获得合适的纳米载体进行药物递送所必需的。按照这种方法,一种新的化合物,在这里表示为化合物 我们在这里选择了一个三官能的α-氨基酸来取代先前制备的基于核糖的Ru(III)配合物的核苷核心。与Ru(III)配合物相连的氨基酸支架被亲水性和亲脂性部分修饰,赋予了在水中形成稳定聚集体的高倾向性,这是获得合适的纳米载体进行药物递送所必需的。按照这种方法,一种新的化合物,在这里表示为化合物I是成功制备和表征的,然后通过动态光散射(DLS),小角中子散射(SANS)和UV-vis与生物相容性阳离子脂质1,2-二醇基-3-三甲基铵丙烷氯化物(DOTAP)共同配制。分析。在一组人类和非人类细胞系上进行了体外评估,它显示出对癌细胞良好的抗增殖活性,IC 50值在μM范围内,并且对用作对照的健康细胞没有相关的细胞毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号