当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of Cu(i) binding to the E2 domain of the amyloid precursor protein – a lesson in quantification of metal binding to proteins via ligand competition†
Metallomics ( IF 2.9 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1039/c7mt00291b Tessa R. Young 1, 2, 3, 4 , Anthony G. Wedd 1, 2, 3, 4 , Zhiguang Xiao 1, 2, 3, 4, 5
Metallomics ( IF 2.9 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1039/c7mt00291b Tessa R. Young 1, 2, 3, 4 , Anthony G. Wedd 1, 2, 3, 4 , Zhiguang Xiao 1, 2, 3, 4, 5
Affiliation
|
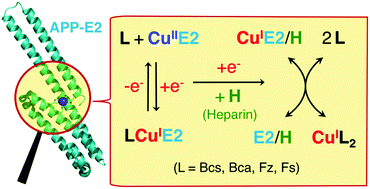
The extracellular domain E2 of the amyloid precursor protein (APP) features a His-rich metal-binding site (denoted as the M1 site). In conjunction with surrounding basic residues, the site participates in interactions with components of the extracellular matrix including heparins, a class of negatively charged polysaccharide molecules of varying length. This work studied the chemistry of Cu(I) binding to APP E2 with the probe ligands Bcs, Bca, Fz and Fs. APP E2 forms a stable Cu(I)-mediated ternary complex with each of these anionic ligands. The complex with Bca was selected for isolation and characterization and was demonstrated, by native ESI-MS analysis, to have the stoichiometry E2 : Cu(I) : Bca = 1 : 1 : 1. Formation of these ternary complexes is specific for the APP E2 domain and requires Cu(I) coordination to the M1 site. Mutation of the M1 site was consistent with the His ligands being part of the E2 ligand set. It is likely that interactions between the negatively charged probe ligands and a positively charged patch on the surface of APP E2 are one aspect of the generation of the stable ternary complexes. Their formation prevented meaningful quantification of the affinity of Cu(I) binding to the M1 site with these probe ligands. However, the ternary complexes are disrupted by heparin, allowing reliable determination of a picomolar Cu(I) affinity for the E2/heparin complex with the Fz or Bca probe ligands. This is the first documented example of the formation of stable ternary complexes between a Cu(I) binding protein and a probe ligand. The ready disruption of the complexes by heparin identified clear ‘tell-tale’ signs for diagnosis of ternary complex formation and allowed a systematic review of conditions and criteria for reliable determination of affinities for metal binding via ligand competition. This study also provides new insights into a potential correlation of APP functions regulated by copper binding and heparin interaction.
中文翻译:

评估Cu(i)与淀粉样前体蛋白E2结构域的结合-通过配体竞争量化金属与蛋白结合的一堂课†
淀粉样蛋白前体蛋白(APP)的胞外域E2具有富含His的金属结合位点(称为M1位点)。该位点与周围的碱性残基结合,参与与细胞外基质(包括肝素)的相互作用,肝素是一类长度可变的带负电荷的多糖分子。这项工作研究了铜(I)与探针配体Bcs,Bca,Fz和Fs结合到APP E2的化学反应。APP E2与这些阴离子配体中的每一个形成稳定的Cu(I)介导的三元络合物。选择了具有Bca的配合物进行分离和表征,并通过天然ESI-MS分析证明其具有化学计量比E2:Cu(I):Bca = 1:1∶1:1。这些三元复合物的形成对于APP E2结构域是特定的,并且需要Cu(I)配位至M1位点。M1位点的突变与His配体是E2配体组的一部分一致。带负电荷的探针配体与APP E2表面带正电荷的贴剂之间的相互作用可能是稳定的三元复合物生成的一方面。它们的形成阻止了有意义的量化的Cu(I)与这些探针配体结合到M1位点的亲和力。但是,三元复合物会被肝素破坏,从而可以可靠地测定皮摩尔Cu(I)对F2 / Bca探针配体对E2 /肝素复合物的亲和力。这是在Cu(I)结合蛋白和探针配体之间形成稳定的三元复合物的第一个记录的例子。肝素对复合物的破坏已经为诊断三元复合物形成确定了清晰的“叙述”迹象,并允许系统地审查条件和标准,以可靠地确定通过配体竞争进行金属结合的亲和力。这项研究还为铜结合和肝素相互作用调节APP功能的潜在相关性提供了新的见解。
更新日期:2017-11-28
中文翻译:

评估Cu(i)与淀粉样前体蛋白E2结构域的结合-通过配体竞争量化金属与蛋白结合的一堂课†
淀粉样蛋白前体蛋白(APP)的胞外域E2具有富含His的金属结合位点(称为M1位点)。该位点与周围的碱性残基结合,参与与细胞外基质(包括肝素)的相互作用,肝素是一类长度可变的带负电荷的多糖分子。这项工作研究了铜(I)与探针配体Bcs,Bca,Fz和Fs结合到APP E2的化学反应。APP E2与这些阴离子配体中的每一个形成稳定的Cu(I)介导的三元络合物。选择了具有Bca的配合物进行分离和表征,并通过天然ESI-MS分析证明其具有化学计量比E2:Cu(I):Bca = 1:1∶1:1。这些三元复合物的形成对于APP E2结构域是特定的,并且需要Cu(I)配位至M1位点。M1位点的突变与His配体是E2配体组的一部分一致。带负电荷的探针配体与APP E2表面带正电荷的贴剂之间的相互作用可能是稳定的三元复合物生成的一方面。它们的形成阻止了有意义的量化的Cu(I)与这些探针配体结合到M1位点的亲和力。但是,三元复合物会被肝素破坏,从而可以可靠地测定皮摩尔Cu(I)对F2 / Bca探针配体对E2 /肝素复合物的亲和力。这是在Cu(I)结合蛋白和探针配体之间形成稳定的三元复合物的第一个记录的例子。肝素对复合物的破坏已经为诊断三元复合物形成确定了清晰的“叙述”迹象,并允许系统地审查条件和标准,以可靠地确定通过配体竞争进行金属结合的亲和力。这项研究还为铜结合和肝素相互作用调节APP功能的潜在相关性提供了新的见解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号