当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral Phosphoric Acid Catalyzed Enantioselective Friedel‐Crafts Reaction of N‐Protected 4‐Aminoindoles with β,γ‐Unsaturated α‐Ketimino Esters
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-12-12 , DOI: 10.1002/adsc.201701354 Yu-Yang Ding 1, 2 , Deng-Feng Xu 1 , Shan-Shui Meng 3 , Yang Li 4 , Jun-Ling Zhao 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-12-12 , DOI: 10.1002/adsc.201701354 Yu-Yang Ding 1, 2 , Deng-Feng Xu 1 , Shan-Shui Meng 3 , Yang Li 4 , Jun-Ling Zhao 3
Affiliation
|
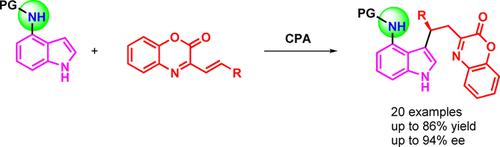
Through a hydrogen bonding guided substrate modification strategy, we have developed a chiral phosphoric acid catalyzed highly enantioselective Friedel‐Crafts reaction of N‐protected 4‐aminoindoles with β,γ‐unsaturated α‐ketimino esters. The mechanism and origins of the enantioselectivity of this process are indentified using DFT calculations. Both the experimental and calculation results indicated that the NH moiety at the indole C4 position is essential for the stereocontrol of this reaction. To further examine the potential usefulness of this method, gram scale synthesis and derivatizations of one of the products were also carried out.
中文翻译:

N-保护的4-氨基吲哚与β,γ-不饱和α-Ketimino酯的手性磷酸催化对映选择性Friedel-Crafts反应
通过氢键引导的底物修饰策略,我们开发了手性磷酸催化N保护的4-氨基吲哚与β,γ-不饱和α-酮亚胺基酯的高对映选择性Friedel-Crafts反应。使用DFT计算确定了该过程对映选择性的机理和起源。实验和计算结果均表明,在吲哚C4位的NH部分对于该反应的立体控制是必不可少的。为了进一步检验该方法的潜在用途,还进行了克级合成和一种产品的衍生化。
更新日期:2017-12-12
中文翻译:

N-保护的4-氨基吲哚与β,γ-不饱和α-Ketimino酯的手性磷酸催化对映选择性Friedel-Crafts反应
通过氢键引导的底物修饰策略,我们开发了手性磷酸催化N保护的4-氨基吲哚与β,γ-不饱和α-酮亚胺基酯的高对映选择性Friedel-Crafts反应。使用DFT计算确定了该过程对映选择性的机理和起源。实验和计算结果均表明,在吲哚C4位的NH部分对于该反应的立体控制是必不可少的。为了进一步检验该方法的潜在用途,还进行了克级合成和一种产品的衍生化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号