当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthetic piperine amide analogs with antimycobacterial activity
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2017-11-27 00:45:51 , DOI: 10.1111/cbdd.13140 Irena Philipova 1 , Violeta Valcheva 2 , Rositsa Mihaylova 3 , Mina Mateeva 3 , Irini Doytchinova 3 , Georgi Stavrakov 1, 3
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2017-11-27 00:45:51 , DOI: 10.1111/cbdd.13140 Irena Philipova 1 , Violeta Valcheva 2 , Rositsa Mihaylova 3 , Mina Mateeva 3 , Irini Doytchinova 3 , Georgi Stavrakov 1, 3
Affiliation
|
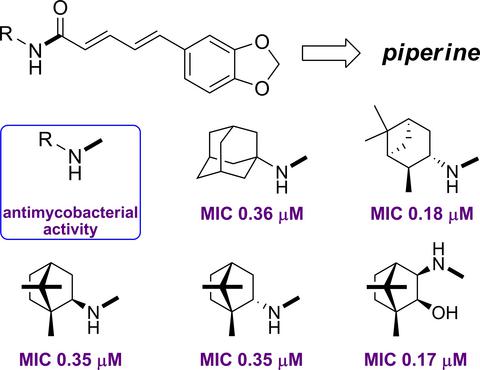
A series of Piperine amide analogues are synthesized by replacing piperidine moiety with different types of cyclic amines, including cyclohexyl, bicyclo[2.2.1]heptyl, adamantyl, and monoterpene-derived fragments. The hybrid analogues with 1-adamantyl and the monoterpene fragments displayed nanomolar antimycobacterial activity with low cytotoxicity against human cells. A QSAR study pointed out the presence of quaternary carbon atom as main structural requirement for the activity. The most promising compound is the (+)-isopinocampheylamine-derived amide, with selectivity index of 1387.8.
中文翻译:

具有抗分枝杆菌活性的合成胡椒碱酰胺类似物
通过用不同类型的环胺(包括环己基,双环[2.2.1]庚基,金刚烷基和单萜衍生的片段)取代哌啶部分,可以合成一系列哌啶酰胺类似物。具有1-金刚烷基和单萜片段的杂合类似物表现出纳摩尔抗分枝杆菌活性,对人细胞的细胞毒性低。一项QSAR研究指出,季碳原子的存在是该活性的主要结构要求。最有前途的化合物是(+)-异opincampcampylylamine衍生的酰胺,选择性指数为1387.8。
更新日期:2017-11-27
中文翻译:

具有抗分枝杆菌活性的合成胡椒碱酰胺类似物
通过用不同类型的环胺(包括环己基,双环[2.2.1]庚基,金刚烷基和单萜衍生的片段)取代哌啶部分,可以合成一系列哌啶酰胺类似物。具有1-金刚烷基和单萜片段的杂合类似物表现出纳摩尔抗分枝杆菌活性,对人细胞的细胞毒性低。一项QSAR研究指出,季碳原子的存在是该活性的主要结构要求。最有前途的化合物是(+)-异opincampcampylylamine衍生的酰胺,选择性指数为1387.8。











































 京公网安备 11010802027423号
京公网安备 11010802027423号