当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isotopically-Labeled Iodoacetamide-Alkyne Probes for Quantitative Cysteine-Reactivity Profiling
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-25 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00832 Masahiro Abo 1 , Chun Li 1 , Eranthie Weerapana 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-25 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00832 Masahiro Abo 1 , Chun Li 1 , Eranthie Weerapana 1
Affiliation
|
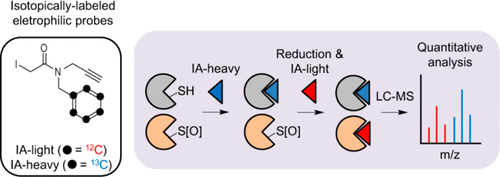
Cysteine residues on proteins serve a variety of catalytic and regulatory functions due to the high nucleophilicity and redox activity of the thiol group. Quantitative proteomic platforms for profiling cysteine reactivity can provide valuable information related to the post-translational modification state and inhibitor occupancy of functional cysteine residues within a complex proteome. Cysteine-reactivity profiling typically monitors changes in the extent of cysteine labeling by cysteine-reactive chemical probes, such as iodoacetamide (IA)-alkyne. To enable accurate measurements of cysteine reactivity changes, isotopic labels are introduced into the two proteomes of interest using either isotopically tagged proteomes (SILAC) or cleavable linkers (isoTOP-ABPP) that are installed using copper-catalyzed azide–alkyne cycloaddition (CuAAC). Here we provide an alternative strategy for isotopic tagging of two proteomes for cysteine-reactivity profiling by developing IA-light and IA-heavy, a pair of isotopically labeled iodoacetamide-alkyne probes. These probes can be utilized for proteome samples that are not amenable to SILAC labeling and are facile to synthesize, especially when compared to the isotopically tagged cleavable linkers. We confirm the quantitative accuracy of IA-light and IA-heavy by assessing cysteine reactivity in a purified thioredoxin protein, as well as globally within a complex proteome where IA-light treatment generates mass-spectrometry identification of 992 cysteine residues. Importantly, these isotopically tagged probes can also be utilized for quantifying the percentage of cysteine modification within a single sample. Preliminary data supports the use of these tags to quantify the stoichiometry of TCEP-susceptible cysteine oxidation events in cell lysates.
中文翻译:

同位素标记的碘乙酰胺-炔烃探针用于定量半胱氨酸反应性分析
由于巯基的高度亲核性和氧化还原活性,蛋白质上的半胱氨酸残基具有多种催化和调节功能。用于定量半胱氨酸反应性的定量蛋白质组学平台可以提供与复杂蛋白质组中功能性半胱氨酸残基的翻译后修饰状态和抑制剂占有率有关的有价值的信息。半胱氨酸反应性分析通常通过半胱氨酸反应性化学探针(例如碘乙酰胺(IA)-炔烃)来监测半胱氨酸标记程度的变化。为了能够精确测量半胱氨酸反应性的变化,使用同位素标记的蛋白质组(SILAC)或可裂解的连接体(isoTOP-ABPP)将同位素标记引入到两个目标蛋白质组中,而同位素标记的蛋白质组是通过铜催化的叠氮化物-炔烃环加成反应(CuAAC)安装的。在这里,我们通过开发IA-light和IA-heavy(一对同位素标记的碘乙酰胺-炔烃探针),为半胱氨酸反应谱分析的两个蛋白质组进行同位素标记提供了另一种策略。这些探针可用于不适合SILAC标记且易于合成的蛋白质组样品,特别是与同位素标记的可裂解接头相比时。我们通过评估纯化的硫氧还蛋白中的半胱氨酸反应性以及整个复杂蛋白质组中的半胱氨酸反应性,来确定IA-light和IA-heavy的定量准确性,其中IA-light处理产生992个半胱氨酸残基的质谱鉴定。重要的是,这些同位素标记的探针也可用于定量单个样品中半胱氨酸修饰的百分比。
更新日期:2017-11-25
中文翻译:

同位素标记的碘乙酰胺-炔烃探针用于定量半胱氨酸反应性分析
由于巯基的高度亲核性和氧化还原活性,蛋白质上的半胱氨酸残基具有多种催化和调节功能。用于定量半胱氨酸反应性的定量蛋白质组学平台可以提供与复杂蛋白质组中功能性半胱氨酸残基的翻译后修饰状态和抑制剂占有率有关的有价值的信息。半胱氨酸反应性分析通常通过半胱氨酸反应性化学探针(例如碘乙酰胺(IA)-炔烃)来监测半胱氨酸标记程度的变化。为了能够精确测量半胱氨酸反应性的变化,使用同位素标记的蛋白质组(SILAC)或可裂解的连接体(isoTOP-ABPP)将同位素标记引入到两个目标蛋白质组中,而同位素标记的蛋白质组是通过铜催化的叠氮化物-炔烃环加成反应(CuAAC)安装的。在这里,我们通过开发IA-light和IA-heavy(一对同位素标记的碘乙酰胺-炔烃探针),为半胱氨酸反应谱分析的两个蛋白质组进行同位素标记提供了另一种策略。这些探针可用于不适合SILAC标记且易于合成的蛋白质组样品,特别是与同位素标记的可裂解接头相比时。我们通过评估纯化的硫氧还蛋白中的半胱氨酸反应性以及整个复杂蛋白质组中的半胱氨酸反应性,来确定IA-light和IA-heavy的定量准确性,其中IA-light处理产生992个半胱氨酸残基的质谱鉴定。重要的是,这些同位素标记的探针也可用于定量单个样品中半胱氨酸修饰的百分比。











































 京公网安备 11010802027423号
京公网安备 11010802027423号