Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2017-11-16 , DOI: 10.1016/j.jfluchem.2017.11.006 Helio G. Bonacorso , Tainara P. Calheiro , Bernardo A. Iglesias , Carolina Hahn da Silveira , Eufrânio N. da Silva Júnior , Alex Ketzer , Fabrício Bublitz , Nilo Zanatta , Marcos A.P. Martins
|
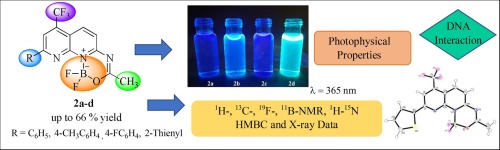
This paper reports the results of the synthesis and structural elucidation by multinuclear NMR spectroscopy and single crystal X-ray diffraction of a new series of four examples of 1,1-difluoro-3-methyl-9-(aryl/heteroaryl)-7-(trifluoromethyl)-1H-[1,3,5,2]oxadiazaborinino[3,4-a][1,8]naphthyridin-11-ium-1-uides, which were obtained, at good yields (60–66%), from the reaction of 7-substituted N-(5-(trifluoromethyl)-1,8-naphthyridin-2-yl)acetamides — in which the 7-substituents are C6H5, 4-CH3C6H4, 4-FC6H4, and 2-Thienyl — with BF3·Et2O solution. One-dimensional multinuclear NMR spectroscopy (1H, 13C, 19F, and 11B) and two-dimensional 1H–15N HMBC are presented as powerful tools for an easy and secure NMR chemical shift assignments and structural characterization of fluorinated 1,8-naphthyridine-based boron complexes. Additionally, investigations of photophysical, electrochemical and DNA-binding properties were done.
中文翻译:

氟化的1,8-萘啶基硼杂环的多核NMR光谱,光物理,电化学和DNA结合特性
本文通过多核NMR光谱和单晶X射线衍射报告了一系列新的1,1-二氟-3-甲基-9-(芳基/杂芳基)-7-的四个实例的合成和结构解析结果。 (三氟甲基)-1 H- [1,3,5,2]恶二氮杂硼酸酯[3,4- a ] [1,8]萘啶11 -1--1-铀,收率良好(60-66 %),由7个取代的N-(5-(三氟甲基)-1,8-萘啶-2-基)乙酰胺的反应制得,其中7个取代基为C 6 H 5,4 -CH 3 C 6 H 4,4 -FC 6 H 4和2-噻吩基-BF 3 · Et2 O溶液。一维多核NMR光谱(1 H,13 C,19 F和11 B)和二维1 H– 15 N HMBC被认为是强大而有效的工具,可轻松,安全地进行NMR化学位移分配和氟化1的结构表征。 ,8-萘啶基硼配合物。此外,还进行了光物理,电化学和DNA结合特性的研究。









































 京公网安备 11010802027423号
京公网安备 11010802027423号