Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2017-03-06 , DOI: 10.1016/j.jfluchem.2017.03.003 Nico Santschi , Thomas Nauser
|
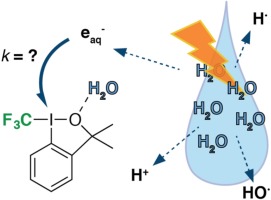
We report on the rate of reduction of a popular electrophilic trifluoromethylating agent, Togni’s reagent (T-CF3), with solvated electrons (eaq−) generated by pulse radiolysis. By means of competition experiments against methyl viologen (MV2+) and direct observation of the decay of eaq− we determined k(T-CF3 + eaq−) ≈ 2 × 1010 M−1 s−1. To the best of our knowledge, this constitutes the first report on a reduction of T-CF3 with an unambiguously clear outer-sphere mechanism. Furthermore, we studied the oxidation of 2-(2-iodophenyl)propan-2-ol (ROH) by peroxyl-radicals to a presumably cyclic iodanyl radical RI. This species RI
was not detected during the reduction of T-CF3 with eaq− and therefore, this reduction does not proceed via heterolytic I-CF3 bond cleavage to CF3− and RI
. More likely, a CF3 radical is formed, as was observed in numerous synthesis studies reported to date.
中文翻译:

Togni试剂的单电子还原速率
我们关于减少一个流行的电子三氟甲基化剂的速率报告,TOGNI试剂(T-CF 3)中,用溶剂化电子(e水溶液- )通过脉冲辐解生成。通过针对甲基紫精(MV竞争实验手段2+)和电子衰减的直接观察水溶液-我们确定ķ(T-CF 3 + E水溶液-)≈2×10 10中号-1小号-1。据我们所知,这是关于T-CF 3还原的第一个报告,其外部明确无误球机制。此外,我们研究了过氧自由基将2-(2-碘苯基)丙-2-醇(ROH)氧化为大概环状的碘丹基RI 。这一物种RI
T-CF的还原过程中未检测到3随e水溶液-并且因此,这种降低不经由异裂I-CF继续进行3键断裂以CF 3 -和RI
。如迄今为止报道的许多合成研究中所观察到的,形成CF 3自由基的可能性更大。











































 京公网安备 11010802027423号
京公网安备 11010802027423号