Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-11-23 , DOI: 10.1016/j.mcat.2017.11.019 Jin Cheng , Yong Song , Qing Ye , Shuiyuan Cheng , Tianfang Kang , Hongxing Dai
|
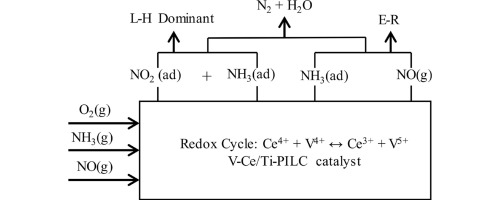
The Ti-pillared clay-supported vanadia − ceria (yV4Ce/Ti-PILC, y was the V weight percentage (wt%), Ce weight percentage = 4 wt%) samples were prepared using the impregnation method. The catalytic activity for the selective catalytic reduction of NOx with NH3 (NH3-SCR) decreased in the order of 1V4Ce/Ti-PILC > 4Ce/Ti-PILC > 2V4Ce/Ti-PILC > 1 V/Ti-PILC > 1V4Ce/Clay > Ti-PILC > Clay, with the 1V4Ce/Ti-PILC sample exhibiting the best activity (NOx conversion >90% at 280–450 °C). Physiochemical properties of the samples were investigated by means of XRD, N2 absorption-desorption, SEM, H2-TPR, NH3-TPD, XPS, and in situ DRIFT techniques. The vanadia was uniformly dispersed on the Ti-PILC support, while the ceria were mainly present in CeO2. The Ti-pillared support was suitable for dispersing the V-Ce composite oxides. The strong interaction between vanadia and ceria led to increased H2 consumption and high-temperature reduction. The Ce4+/Ce3+ and V5+/V4+ redox cycle (V4+ + Ce4+ ↔ V5+ + Ce3+) accounted for the excellent NH3-SCR catalytic performance of the 1V4Ce/Ti-PILC sample. The doping of a proper amount of vanadia could promote formation of Ce3+ on the surface of 1V4Ce/Ti-PILC, and the formed Ce3+ could generate a more amount of the chemisorbed oxygen species that favored the NH3-SCR reaction. Furthermore, the 1V4Ce/Ti-PILC sample possessed high catalytic acidity and strong NH3 adsorption ability, which were responsible for high NO conversion in a wide range of temperatures. The NH3-SCR reaction on the 4Ce/Ti-PILC and 1V4Ce/Ti-PILC samples obeyed the Langmuir–Hinshelwood (L–H) and Eley-Rideal (E–R) mechanisms, with the former being dominant.
中文翻译:

在V-Ce / Ti-PILC催化剂上用氨选择性催化还原NO的机理研究
使用浸渍法制备了Ti柱状粘土负载的钒-二氧化铈(y V4Ce / Ti-PILC,y为V的重量百分比(wt%),Ce的重量百分比= 4 wt%)样品。用于选择性催化还原的催化活性NO X与NH 3(NH 3 -SCR)在1V4Ce / Ti系PILC> 4CE / Ti系PILC> 2V4Ce / Ti系PILC的顺序降低> 1个V / Ti系PILC> 1V4Ce /粘土>的Ti-PILC>黏土,与1V4Ce / Ti系PILC样品显示出最佳活性(NO X转化率> 90%的在280-450℃)。通过XRD,N 2吸收-解吸,SEM,H 2 -TPR,NH 3考察了样品的理化性质。-TPD,XPS和原位DRIFT技术。钒氧化物均匀地分散在Ti-PILC载体上,而二氧化铈主要存在于CeO 2中。Ti柱状载体适合于分散V-Ce复合氧化物。钒与二氧化铈之间的强相互作用导致H 2消耗增加和高温降低。Ce的4+ / CE 3+和V 5+ / V 4+的氧化还原循环(V 4+ + Ce的4+ ↔V 5+ +的Ce 3+)占优良NH 3的1V4Ce /钛-SCR催化性能-PILC样品。掺杂适量的钒可以促进铈的形成1V4Ce / Ti-PILC表面存在3+,而形成的Ce 3+可产生更多的化学吸附氧,有利于NH 3 -SCR反应。此外,1V4Ce / Ti-PILC样品具有较高的催化酸度和较强的NH 3吸附能力,这是在宽温度范围内实现高NO转化的原因。4Ce / Ti-PILC和1V4Ce / Ti-PILC样品上的NH 3 -SCR反应服从Langmuir-Hinshelwood(L-H)和Eley-Rideal(E-R)机理,前者占主导地位。











































 京公网安备 11010802027423号
京公网安备 11010802027423号