Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-11-23 , DOI: 10.1016/j.mcat.2017.11.023 Michael A. Jackson , Mark G. White , Richard T. Haasch , Steven C. Peterson , Judith A. Blackburn
|
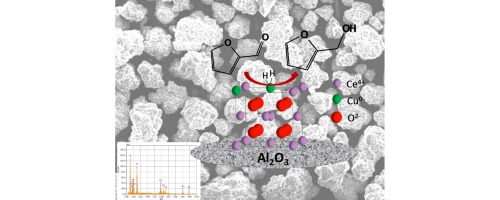
The hydrogenation of furfural to furfuryl alcohol over a CuOCeO2/ɣ-Al2O3 catalyst in a flow reactor is reported. The catalyst was prepared by the wet impregnation of Cu onto a CeO2/ɣ-Al2O3 precursor. The calcined catalyst was then treated with HNO3 to remove surface CuO resulting in a mixed CuCe oxide supported on ɣ-Al2O3. The catalyst was characterized by BET surface measurements, powder XRD, EDX, XPS, and H2-TPR. Reduction of the catalyst results in migration of Cu2O to the surface of the solid solution. The CeO2 facilitates the reduction of the Cu which in turn leads to increased activated hydrogen being available at the reaction temperature. Furfural hydrogenation was carried out in a downflow reactor at 175 °C under hydrogen pressures ranging from 1 to 10 bar. Selectivity to furfuryl alcohol and time on stream before loss of activity due to coke formation were greater at higher pressure. Coke formation was greater at higher furfural flow rates indicating a competition exists between hydrogenation and coke formation. The side products that were measured included 2-methylfuran and difurfuryl ether. The kinetics of the reduction of the Cu sites are also described and found to contribute to catalyst activity. Comparison to commercial copper chromite and 5 wt% Cu/Al2O3 were made to assess practical application of this catalyst.
中文翻译:

气相填充床反应器中CuOCeO 2 / Al 2 O 3的动态Cu表面的糠醛加氢
糠醛糠醇在CuOCeO氢化2 /ɣ-Al系2 ö 3报道催化剂在流动反应器。催化剂通过Cu的湿浸渍制备到的CeO 2 /ɣ-Al系2 ö 3前体。煅烧后的催化剂随后用HNO处理3,以除去导致支撑在ɣ -铝混合氧化物CuCe表面的CuO 2 ö 3。通过BET表面测量,粉末XRD,EDX,XPS和H 2 -TPR来表征催化剂。催化剂的还原导致Cu 2 O迁移到固溶体的表面。首席执行官2促进铜的还原,这又导致在反应温度下可利用的活性氢增加。糠醛加氢在175°C的下流反应器中于1至10 bar的氢气压力下进行。在较高压力下,对糠醇的选择性和由于焦炭形成而导致的活性损失之前的生产时间更长。在较高的糠醛流速下,焦炭形成更大,表明氢化和焦炭形成之间存在竞争。测量的副产物包括2-甲基呋喃和二糠基醚。还描述了Cu位点还原的动力学,发现该动力学有助于催化剂活性。与市售亚铬酸铜和5 wt%Cu / Al 2 O 3的比较 进行评估该催化剂的实际应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号