Journal of Catalysis ( IF 6.5 ) Pub Date : 2017-11-23 , DOI: 10.1016/j.jcat.2017.11.008 Kun Zhao , Yan Su , Xie Quan , Yanming Liu , Shuo Chen , Hongtao Yu
|
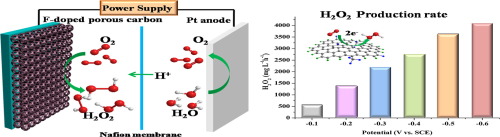
Electrochemical synthesis of hydrogen peroxide (H2O2) via two-electron pathway of oxygen reduction reaction is a promising alternative to the current anthraquinone process. The H2O2 production from O2 is a competing reaction with four-electron O2 reduction to H2O, and the selectivity is related to the adsorption energy of the OOH intermediate on electrocatalysts surface. Generally, the properties for binding of OOH intermediate on catalysts can be controlled by changing its electronic structure. Herein, the electronic structure of porous carbon materials was tuned by doping different types and contents of fluorine species. The yield of H2O2 generation depended on the F content and the best catalytic activity toward H2O2 electrosynthesis was obtained with F content of 3.41 at.%. The resultant F-doped porous carbon (FPC) catalysts exhibited good H2O2 selectivity of 97.5–83.0% and the H2O2 production rate could reach 112.6–792.6 mmol h−1 g−1 over the potential range of 0.2 V to −0.3 vs. RHE (pH 1). The density functional theory (DFT) calculations and experiments revealed that the incorporation of CF2, 3 into carbon plane promotes the activation of O2 molecule and facilitates desorption of OOH intermediate, which was crucial to H2O2 synthesis.
中文翻译:

通过在氟掺杂的分层多孔碳上选择性地还原O 2来增强H 2 O 2的产生
通过氧还原反应的两电子途径电化学合成过氧化氢(H 2 O 2)是当前蒽醌工艺的一种有前途的替代方法。为H 2 ö 2选自O生产2是一个具有四个电子○竞争反应2还原成H 2 O,并且选择性是关系到OOH上电催化剂表面中间的吸附能。通常,可以通过改变其电子结构来控制OOH中间体在催化剂上的结合性能。在此,通过掺杂不同类型和含量的氟物种来调节多孔碳材料的电子结构。H 2的收率O 2的生成取决于F的含量,并且以3.41 at。%的F含量获得了对H 2 O 2电合成的最佳催化活性。所得的F掺杂多孔碳(FPC)催化剂表现出良好的H 2 O 2选择性,为97.5–83.0%,并且在0.2 V的电位范围内,H 2 O 2的生产率可达到112.6–792.6 mmol h -1 g -1。相对于RHE(pH 1)为-0.3。密度泛函理论(DFT)的计算和实验表明,将CF 2、3引入碳平面可促进O 2的活化。分子并促进OOH中间体的解吸,这对H 2 O 2的合成至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号