Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2017-11-21 , DOI: 10.1016/j.bioorg.2017.11.014 Alexander Sapegin , Stanislav Kalinin , Andrea Angeli , Claudiu T. Supuran , Mikhail Krasavin
|
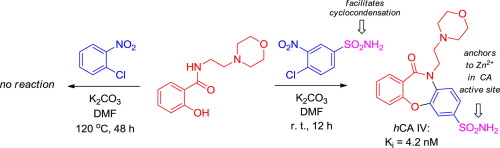
4-Chloro-3-nitrobenzenesulfonamide reacted cleanly at room-temperature with a range of bis-electrophilic phenols bearing an NH-acidic functionality (secondary carboxamide or pyrazole) in the ortho-position. This produced a novel class of [1,4]oxazepine-based primary sulfonamides which exhibited strong inhibition of therapeutically relevant human carbonic anhydrases. 2-Chloronitrobenzene did not enter a similar cyclocondensation process, even under prolonged heating. Thus, the primary sulfonamide functionality plays a dual role by enabling the [1,4]oxazepine ring construction and acting as a enzyme prosthetic zinc-binding group when the resulting [1,4]oxazepine sulfonamides are employed as carbonic anhydrase inhibitors.
中文翻译:

未保护的伯磺酰胺基团可促进成环的级联反应,从而形成基于多环[1,4]氧杂ze庚因的碳酸酐酶抑制剂
4-氯-3-硝基苯磺酰胺在室温下与一系列在邻位带有NH-酸性官能团(仲羧酰胺或吡唑)的双亲电子酚干净地反应。这产生了一类新的基于[1,4]氧杂氮杂的伯磺酰胺,对治疗相关的人类碳酸酐酶表现出强烈的抑制作用。即使长时间加热,2-氯硝基苯也不会进入类似的环缩过程。因此,当将所得的[1,4]氧杂氮杂磺酰胺用作碳酸酐酶抑制剂时,伯磺酰胺官能度通过使[1,4]氧杂氮杂灵环结构和充当酶辅锌结合基团发挥双重作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号