当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic Activation of Charge Transfer in Anionic Redox Process for Li‐Ion Batteries
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2017-11-23 , DOI: 10.1002/adfm.201704864 Biao Li 1 , Ning Jiang 1 , Weifeng Huang 1 , Huijun Yan 1 , Yuxuan Zuo 1 , Dingguo Xia 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2017-11-23 , DOI: 10.1002/adfm.201704864 Biao Li 1 , Ning Jiang 1 , Weifeng Huang 1 , Huijun Yan 1 , Yuxuan Zuo 1 , Dingguo Xia 1
Affiliation
|
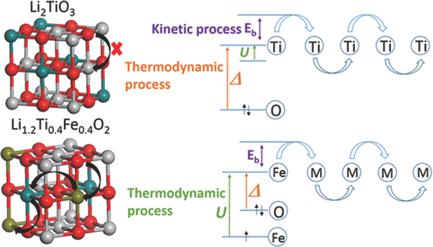
Anionic redox processes are vital to realize high capacity in lithium‐rich electrodes of lithium‐ion batteries. However, the activation mechanism of these processes remains ambiguous, hampering further implementation in new electrode design. This study demonstrates that the electrochemical activity of inert cubic‐Li2TiO3 is triggered by Fe3+ substitution, to afford considerable oxygen redox activity. Coupled with first principles calculations, it is found that electron holes tend to be selectively generated on oxygen ions bonded to Fe rather than Ti. Subsequently, a thermodynamic threshold is unravelled dictated by the relative values of the Coulomb and exchange interactions (U) and charge‐transfer energy (Δ) for the anionic redox electron‐transfer process, which is further verified by extension to inactive layered Li2TiS3, in which the sulfur redox process is activated by Co substitution to form Li1.2Ti0.6Co0.2S2. This work establishes general guidance for the design of high‐capacity electrodes utilizing anionic redox processes.
中文翻译:

锂离子电池阴离子氧化还原过程中电荷转移的热力学激活
阴离子氧化还原工艺对于在锂离子电池的富锂电极中实现高容量至关重要。然而,这些过程的激活机制仍然是模棱两可的,妨碍了在新电极设计中的进一步实施。这项研究表明,惰性立方-Li 2 TiO 3的电化学活性是由Fe 3+取代触发的,具有相当大的氧还原活性。结合第一原理计算,发现电子空穴倾向于在结合到Fe而不是Ti的氧离子上选择性地产生。随后,根据库仑和交换相互作用的相对值(U)和阴离子氧化还原电子转移过程的电荷转移能量(Δ),这可以通过扩展到非活性层状Li 2 TiS 3来进一步验证,其中通过Co取代激活硫氧化还原过程以形成Li 1.2 Ti 0.6 Co 0.2 S 2。这项工作为利用阴离子氧化还原工艺设计高容量电极建立了一般指导。
更新日期:2017-11-23
中文翻译:

锂离子电池阴离子氧化还原过程中电荷转移的热力学激活
阴离子氧化还原工艺对于在锂离子电池的富锂电极中实现高容量至关重要。然而,这些过程的激活机制仍然是模棱两可的,妨碍了在新电极设计中的进一步实施。这项研究表明,惰性立方-Li 2 TiO 3的电化学活性是由Fe 3+取代触发的,具有相当大的氧还原活性。结合第一原理计算,发现电子空穴倾向于在结合到Fe而不是Ti的氧离子上选择性地产生。随后,根据库仑和交换相互作用的相对值(U)和阴离子氧化还原电子转移过程的电荷转移能量(Δ),这可以通过扩展到非活性层状Li 2 TiS 3来进一步验证,其中通过Co取代激活硫氧化还原过程以形成Li 1.2 Ti 0.6 Co 0.2 S 2。这项工作为利用阴离子氧化还原工艺设计高容量电极建立了一般指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号