当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dual Location, Dual Acidic pH/Reduction-Responsive Degradable Block Copolymer: Synthesis and Investigation of Ketal Linkage Instability under ATRP Conditions
Macromolecules ( IF 5.5 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.macromol.7b02070 Arman Moini Jazani 1 , Jung Kwon Oh 1
Macromolecules ( IF 5.5 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.macromol.7b02070 Arman Moini Jazani 1 , Jung Kwon Oh 1
Affiliation
|
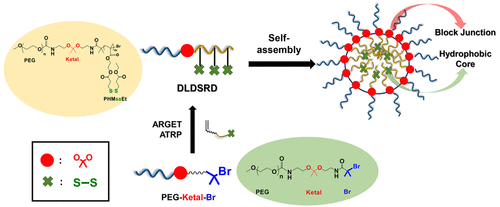
Stimuli-responsive degradation (SRD) undergoing chemical transition through the cleavage of labile linkages has been proved to dramatically increase the versatility of stimuli-responsive block copolymers. In particular, dual or multiple stimuli-responsive degradable block copolymers that can be triggered by two endogenous stimuli of acidic pH and reduction are in high demand. Here, a new strategy utilizing atom transfer radical polymerization (ATRP) is reported to synthesize a dual acidic pH/reduction-responsive degradable block copolymer (DLDSRD) labeled with an acidic pH-labile ketal linkage at the block junction and pendant reductively cleavable disulfide groups in hydrophobic block at dual locations. A robust route with multiple steps utilizing carbamate chemistry to endow stability during protection/deprotection steps enables the synthesis of a novel poly(ethylene glycol)-based ATRP macroinitiator labeled with a ketal linkage (PEG-ketal-Br macroinitiator). Conducting ATRP allows for the synthesis of a series of DLDSRD diblock copolymers consisting of a hydrophilic poly(ethylene glycol) block covalently conjugated through a ketal linkage with a hydrophobic polymethacrylate block having multiple disulfide pendants. Analysis shows an unexpectedly high degree of polymerization of the hydrophobic polymethacrylate block that could be attributed to the instability of ketal linkages under ATRP conditions. The preliminary results from aqueous micellization and dual acidic pH/reduction-responsive cleavage of ketal and disulfide linkages suggest the feasibility of DLDSRD-based nanoassemblies toward effective drug delivery exhibiting precisely controlled release in response to dual stimuli at dual locations (core and interfaces).
中文翻译:

双位置,双酸性pH /还原反应性可降解嵌段共聚物:ATRP条件下缩合键的不稳定性的合成和研究
通过不稳定键的断裂经历化学转变的刺激响应性降解(SRD)已被证明可以显着提高刺激响应性嵌段共聚物的多功能性。尤其是,迫切需要可由两种内源性酸性pH值刺激和还原引发的双或多种刺激响应性可降解嵌段共聚物。在这里,据报道,利用原子转移自由基聚合(ATRP)的新策略可合成在嵌段连接处带有酸性pH不稳定缩酮键标记的侧酸性pH /还原反应性可降解嵌段共聚物(DLDSRD),以及侧基可还原裂解的二硫键在两个位置的疏水嵌段中。利用氨基甲酸酯化学在保护/去保护步骤中赋予稳定性的多步骤稳健路线使得能够合成标记有缩酮键的新型基于聚乙二醇的ATRP大分子引发剂(PEG-缩酮-Br大分子引发剂)。进行ATRP可以合成一系列DLDSRD二嵌段共聚物,该共聚物由通过缩酮键与具有多个二硫键侧基的疏水性聚甲基丙烯酸酯嵌段共价共轭的亲水性聚乙二醇嵌段组成。分析表明,疏水性聚甲基丙烯酸酯嵌段的聚合度出乎意料地高,这可能归因于ATRP条件下缩酮键的不稳定性。
更新日期:2017-11-22
中文翻译:

双位置,双酸性pH /还原反应性可降解嵌段共聚物:ATRP条件下缩合键的不稳定性的合成和研究
通过不稳定键的断裂经历化学转变的刺激响应性降解(SRD)已被证明可以显着提高刺激响应性嵌段共聚物的多功能性。尤其是,迫切需要可由两种内源性酸性pH值刺激和还原引发的双或多种刺激响应性可降解嵌段共聚物。在这里,据报道,利用原子转移自由基聚合(ATRP)的新策略可合成在嵌段连接处带有酸性pH不稳定缩酮键标记的侧酸性pH /还原反应性可降解嵌段共聚物(DLDSRD),以及侧基可还原裂解的二硫键在两个位置的疏水嵌段中。利用氨基甲酸酯化学在保护/去保护步骤中赋予稳定性的多步骤稳健路线使得能够合成标记有缩酮键的新型基于聚乙二醇的ATRP大分子引发剂(PEG-缩酮-Br大分子引发剂)。进行ATRP可以合成一系列DLDSRD二嵌段共聚物,该共聚物由通过缩酮键与具有多个二硫键侧基的疏水性聚甲基丙烯酸酯嵌段共价共轭的亲水性聚乙二醇嵌段组成。分析表明,疏水性聚甲基丙烯酸酯嵌段的聚合度出乎意料地高,这可能归因于ATRP条件下缩酮键的不稳定性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号