当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deconstructing Lipid Kinase Inhibitors by Chemical Proteomics
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.biochem.7b00962 Rebecca L. McCloud 1 , Caroline E. Franks 1 , Sean T. Campbell 1, 2 , Benjamin W. Purow 3 , Thurl E. Harris 4 , Ku-Lung Hsu 1, 4
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.biochem.7b00962 Rebecca L. McCloud 1 , Caroline E. Franks 1 , Sean T. Campbell 1, 2 , Benjamin W. Purow 3 , Thurl E. Harris 4 , Ku-Lung Hsu 1, 4
Affiliation
|
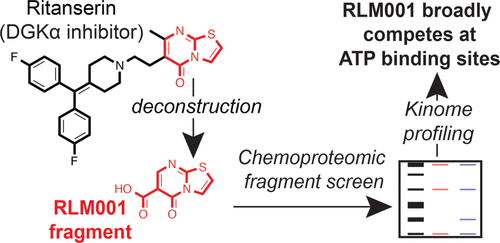
Diacylglycerol kinases (DGKs) regulate lipid metabolism and cell signaling through ATP-dependent phosphorylation of diacylglycerol to biosynthesize phosphatidic acid. Selective chemical probes for studying DGKs are currently lacking and are needed to annotate isoform-specific functions of these elusive lipid kinases. Previously, we explored fragment-based approaches to discover a core fragment of DGK-α (DGKα) inhibitors responsible for selective binding to the DGKα active site. Here, we utilize quantitative chemical proteomics to deconstruct widely used DGKα inhibitors to identify structural regions mediating off-target activity. We tested the activity of a fragment (RLM001) derived from a nucleotide-like region found in the DGKα inhibitors R59022 and ritanserin and discovered that RLM001 mimics ATP in its ability to broadly compete at ATP-binding sites of DGKα as well as >60 native ATP-binding proteins (kinases and ATPases) detected in cell proteomes. Equipotent inhibition of activity-based probe labeling by RLM001 supports a contiguous ligand-binding site composed of C1, DAGKc, and DAGKa domains in the DGKα active site. Given the lack of available crystal structures of DGKs, our studies highlight the utility of chemical proteomics in revealing active-site features of lipid kinases to enable development of inhibitors with enhanced selectivity against the human proteome.
中文翻译:

通过化学蛋白质组学解构脂质激酶抑制剂
二酰基甘油激酶(DGK)通过二酰基甘油的ATP依赖性磷酸化来调节脂质代谢和细胞信号传导,从而生物合成磷脂酸。目前尚缺乏用于研究DGK的选择性化学探针,并且需要使用这些化学探针来注释这些难以捉摸的脂质激酶的同工型特异性功能。以前,我们探索了基于片段的方法来发现负责选择性结合DGKα活性位点的DGK-α(DGKα)抑制剂的核心片段。在这里,我们利用定量化学蛋白质组学来解构广泛使用的DGKα抑制剂,以确定介导脱靶活性的结构区域。我们测试了从DGKα抑制剂R59022和利坦色林中发现的核苷酸样区域衍生的片段(RLM001)的活性,发现RLM001模仿ATP的能力广泛竞争于DGKα的ATP结合位点,以及> 60的天然在细胞蛋白质组中检测到ATP结合蛋白(激酶和ATPase)。RLM001对基于活性的探针标记的等电位抑制支持了由DGKα活性位点中的C1,DAGKc和DAGKa结构域组成的连续配体结合位点。考虑到缺乏可用的DGK晶体结构,我们的研究突出了化学蛋白质组学在揭示脂质激酶活性位点特征方面的实用性,从而能够开发出对人类蛋白质组具有更高选择性的抑制剂。在细胞蛋白质组中检测到60种天然ATP结合蛋白(激酶和ATPase)。RLM001对基于活性的探针标记的等电位抑制支持了由DGKα活性位点中的C1,DAGKc和DAGKa结构域组成的连续配体结合位点。考虑到缺乏可用的DGK晶体结构,我们的研究突出了化学蛋白质组学在揭示脂质激酶活性位点特征方面的实用性,从而能够开发出对人类蛋白质组具有更高选择性的抑制剂。在细胞蛋白质组中检测到60种天然ATP结合蛋白(激酶和ATPase)。RLM001对基于活性的探针标记的等电位抑制支持了由DGKα活性位点中的C1,DAGKc和DAGKa结构域组成的连续配体结合位点。考虑到缺乏可用的DGK晶体结构,我们的研究突出了化学蛋白质组学在揭示脂质激酶活性位点特征方面的实用性,从而能够开发出对人类蛋白质组具有更高选择性的抑制剂。
更新日期:2017-11-23
中文翻译:

通过化学蛋白质组学解构脂质激酶抑制剂
二酰基甘油激酶(DGK)通过二酰基甘油的ATP依赖性磷酸化来调节脂质代谢和细胞信号传导,从而生物合成磷脂酸。目前尚缺乏用于研究DGK的选择性化学探针,并且需要使用这些化学探针来注释这些难以捉摸的脂质激酶的同工型特异性功能。以前,我们探索了基于片段的方法来发现负责选择性结合DGKα活性位点的DGK-α(DGKα)抑制剂的核心片段。在这里,我们利用定量化学蛋白质组学来解构广泛使用的DGKα抑制剂,以确定介导脱靶活性的结构区域。我们测试了从DGKα抑制剂R59022和利坦色林中发现的核苷酸样区域衍生的片段(RLM001)的活性,发现RLM001模仿ATP的能力广泛竞争于DGKα的ATP结合位点,以及> 60的天然在细胞蛋白质组中检测到ATP结合蛋白(激酶和ATPase)。RLM001对基于活性的探针标记的等电位抑制支持了由DGKα活性位点中的C1,DAGKc和DAGKa结构域组成的连续配体结合位点。考虑到缺乏可用的DGK晶体结构,我们的研究突出了化学蛋白质组学在揭示脂质激酶活性位点特征方面的实用性,从而能够开发出对人类蛋白质组具有更高选择性的抑制剂。在细胞蛋白质组中检测到60种天然ATP结合蛋白(激酶和ATPase)。RLM001对基于活性的探针标记的等电位抑制支持了由DGKα活性位点中的C1,DAGKc和DAGKa结构域组成的连续配体结合位点。考虑到缺乏可用的DGK晶体结构,我们的研究突出了化学蛋白质组学在揭示脂质激酶活性位点特征方面的实用性,从而能够开发出对人类蛋白质组具有更高选择性的抑制剂。在细胞蛋白质组中检测到60种天然ATP结合蛋白(激酶和ATPase)。RLM001对基于活性的探针标记的等电位抑制支持了由DGKα活性位点中的C1,DAGKc和DAGKa结构域组成的连续配体结合位点。考虑到缺乏可用的DGK晶体结构,我们的研究突出了化学蛋白质组学在揭示脂质激酶活性位点特征方面的实用性,从而能够开发出对人类蛋白质组具有更高选择性的抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号