当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Recent Advances in Radical-Initiated C(sp3)–H Bond Oxidative Functionalization of Alkyl Nitriles
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1021/acscatal.7b03334 Xue-Qiang Chu 1 , Danhua Ge 1 , Zhi-Liang Shen 1 , Teck-Peng Loh 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1021/acscatal.7b03334 Xue-Qiang Chu 1 , Danhua Ge 1 , Zhi-Liang Shen 1 , Teck-Peng Loh 1, 2
Affiliation
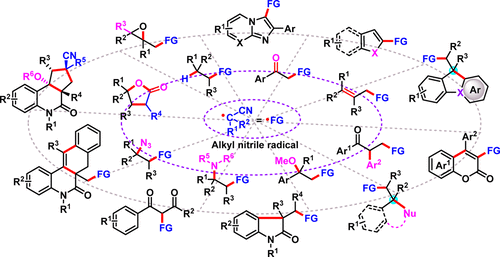
|
Chemoselective functionalizations of intrinsically less reactive C(sp3)–H bonds of alkyl nitriles are of particular interest to the chemical community because these strategies provide opportunities for the introduction of important cyanoalkyl groups onto target frameworks in a step-economical fashion. In recent years, the introduction of nitrile-containing alkyl radicals in tandem radical additions and oxidative couplings has inarguably brought chemists a new radical reaction platform for the diverse synthesis of natural products and pharmaceuticals. Compared with the wide applications of various C-centered radicals adjacent to a heteroatom, however, nitrile-containing alkyl radicals remain largely unexplored. New methods for C(sp3)–H bond oxidative functionalization of alkyl nitriles and new mechanistic manifolds would result in the development of a broad range of novel reactions. Therefore, this review will give an overview of various types of radical cyanoalkylation using the key alkyl nitrile reactants, which lie beyond traditional coupling chemistry.
中文翻译:

烷基腈的自由基引发的C(sp 3)–H键氧化功能化的最新进展
化学界对本质上反应性较低的烷基腈的C(sp 3)–H键的化学选择性官能化特别感兴趣,因为这些策略为将重要的氰基烷基以逐步经济的方式引入目标框架提供了机会。近年来,在串联自由基加成和氧化偶联中引入含腈烷基自由基无疑为化学家带来了一个新的自由基反应平台,可用于天然产物和药物的多种合成。然而,与杂原子相邻的各种以C为中心的自由基的广泛应用相比,含腈的烷基自由基在很大程度上尚未开发。C(sp 3的新方法)–烷基腈的H键氧化功能化和新的机械流形将导致广泛的新型反应的发展。因此,本综述将概述使用关键烷基腈反应物的各种类型的氰基氰基烷基化反应,这超出了传统的偶联化学方法。
更新日期:2017-12-22
中文翻译:

烷基腈的自由基引发的C(sp 3)–H键氧化功能化的最新进展
化学界对本质上反应性较低的烷基腈的C(sp 3)–H键的化学选择性官能化特别感兴趣,因为这些策略为将重要的氰基烷基以逐步经济的方式引入目标框架提供了机会。近年来,在串联自由基加成和氧化偶联中引入含腈烷基自由基无疑为化学家带来了一个新的自由基反应平台,可用于天然产物和药物的多种合成。然而,与杂原子相邻的各种以C为中心的自由基的广泛应用相比,含腈的烷基自由基在很大程度上尚未开发。C(sp 3的新方法)–烷基腈的H键氧化功能化和新的机械流形将导致广泛的新型反应的发展。因此,本综述将概述使用关键烷基腈反应物的各种类型的氰基氰基烷基化反应,这超出了传统的偶联化学方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号