当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiazolium salt-catalyzed [3 + 2 + 1] cyclization for the synthesis of trisubstituted 2-pyrones using arylglyoxals as a carbonyl source
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1039/c7qo00899f Li-Chen Xu 1, 2, 3, 4, 5 , Peng Zhou 1, 2, 3, 4, 5 , Jia-Zhuo Li 1, 2, 3, 4, 5 , Wen-Juan Hao 1, 2, 3, 4, 5 , Shu-Jiang Tu 1, 2, 3, 4, 5 , Bo Jiang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1039/c7qo00899f Li-Chen Xu 1, 2, 3, 4, 5 , Peng Zhou 1, 2, 3, 4, 5 , Jia-Zhuo Li 1, 2, 3, 4, 5 , Wen-Juan Hao 1, 2, 3, 4, 5 , Shu-Jiang Tu 1, 2, 3, 4, 5 , Bo Jiang 1, 2, 3, 4, 5
Affiliation
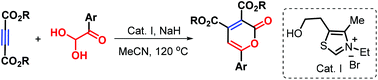
|
A new thiazolium salt-catalyzed [3 + 2 + 1] cyclization of acetylenedicarboxylates with arylglyoxals has been developed, enabling organocatalytic umpolung to access trisubstituted 2-pyrones with good yields via C–C bond cleavage, in which arylglyoxals played dual roles as a ring component as well as a carbonyl source. The reaction mechanism was proposed based on LC-MS analysis.
中文翻译:

噻唑盐催化的[3 + 2 + 1]环化反应,以芳基乙二醛为羰基来源合成三取代的2-吡喃酮
已开发出一种新的噻唑鎓盐催化的乙炔基二羧酸与芳基乙二醛的[3 + 2 + 1]环化反应,使有机催化的umpolung能够通过C–C键裂解获得高收率的三取代2-吡喃酮,其中芳基乙二醛起着环的双重作用。组分以及羰基来源。基于LC-MS分析提出了反应机理。
更新日期:2017-11-23
中文翻译:

噻唑盐催化的[3 + 2 + 1]环化反应,以芳基乙二醛为羰基来源合成三取代的2-吡喃酮
已开发出一种新的噻唑鎓盐催化的乙炔基二羧酸与芳基乙二醛的[3 + 2 + 1]环化反应,使有机催化的umpolung能够通过C–C键裂解获得高收率的三取代2-吡喃酮,其中芳基乙二醛起着环的双重作用。组分以及羰基来源。基于LC-MS分析提出了反应机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号