当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective access to dipyrazolo-fused pyridines via formal [3 + 2 + 1] heteroannulation of methyl ketones with pyrazol-5-amines
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1039/c7qo00941k Qinghe Gao 1, 2, 3, 4, 5 , Shuang He 1, 2, 3, 4 , Xia Wu 5, 6, 7, 8, 9 , Jingjing Zhang 5, 6, 7, 8, 9 , Suping Bai 1, 2, 3, 4 , Yandong Wu 5, 6, 7, 8, 9 , Anxin Wu 5, 6, 7, 8, 9
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1039/c7qo00941k Qinghe Gao 1, 2, 3, 4, 5 , Shuang He 1, 2, 3, 4 , Xia Wu 5, 6, 7, 8, 9 , Jingjing Zhang 5, 6, 7, 8, 9 , Suping Bai 1, 2, 3, 4 , Yandong Wu 5, 6, 7, 8, 9 , Anxin Wu 5, 6, 7, 8, 9
Affiliation
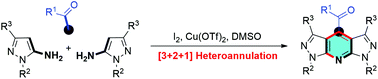
|
A fascinating new form of the Hantzsch reaction with methyl ketones and pyrazol-5-amines for the selective synthesis of dipyrazolo-fused pyridines has been discovered. This special [3 + 2 + 1] heteroannulation was accomplished via the domino generation of two C–C bonds and one C–N bond. Preliminary mechanistic studies indicated that the I2/DMSO system realized the oxidative carbonylation of Csp3–H of methyl ketones, while Cu(OTf)2 acted as a relay reagent to accelerate this transformation.
中文翻译:

通过吡唑-5-胺与甲基酮进行正式的[3 + 2 + 1]杂环化反应,选择性地获得与吡唑并有吡啶的吡啶
已经发现了一种引人入胜的新形式的Hantzsch反应,可与甲基酮和吡唑-5-胺选择性合成吡唑并与吡啶的吡啶。这种特殊的[3 + 2 + 1]杂环化是通过多米诺骨牌产生两个C–C键和一个C–N键来实现的。初步的机理研究表明,I 2 / DMSO系统实现了甲基酮Csp 3 -H的氧化羰基化作用,而Cu(OTf)2充当了中继剂以加速这种转变。
更新日期:2017-11-23
中文翻译:

通过吡唑-5-胺与甲基酮进行正式的[3 + 2 + 1]杂环化反应,选择性地获得与吡唑并有吡啶的吡啶
已经发现了一种引人入胜的新形式的Hantzsch反应,可与甲基酮和吡唑-5-胺选择性合成吡唑并与吡啶的吡啶。这种特殊的[3 + 2 + 1]杂环化是通过多米诺骨牌产生两个C–C键和一个C–N键来实现的。初步的机理研究表明,I 2 / DMSO系统实现了甲基酮Csp 3 -H的氧化羰基化作用,而Cu(OTf)2充当了中继剂以加速这种转变。











































 京公网安备 11010802027423号
京公网安备 11010802027423号