当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pd-Catalyzed regioselective intramolecular dehydrogenative C-5 cross coupling in an N-substituted pyrrole-azole system
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1039/c7ob02676e Krishna N. Tripathi 1, 2, 3, 4, 5 , Devalina Ray 1, 2, 3, 4, 5 , Ravi P. Singh 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1039/c7ob02676e Krishna N. Tripathi 1, 2, 3, 4, 5 , Devalina Ray 1, 2, 3, 4, 5 , Ravi P. Singh 1, 2, 3, 4, 5
Affiliation
|
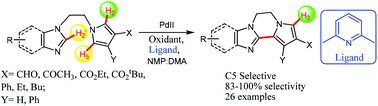
Functionalized polycyclic pyrrole-azole structures possessing fused six membered and seven membered rings were directly synthesized via ligand-enabled, Pd-catalyzed, site selective, intramolecular cross couplings of N-substituted pyrrole-azoles. C5-H activation in the presence of a reactive C2-H remains a challenge that needs to be addressed and this was targeted to be resolved through the present approach by specifically generating the cyclized products with 83–100% selectivity. The featured methodology provides a novel disconnection for the synthesis of pyrrole containing alkaloids and medicinal compounds.
中文翻译:

N取代的吡咯-吡咯体系中Pd催化的区域选择性分子内脱氢C-5交叉偶联
通过N-取代的吡咯-唑的配体赋能的,Pd催化的,位点选择的,分子内的交叉偶联,直接合成了具有稠合的六元环和七元环的官能化多环吡咯-唑结构。在存在反应性C2-H的情况下C5-H的活化仍然是一个需要解决的挑战,并且有针对性地通过本方法解决该问题,具体方法是以83-100%的选择性生成环化产物。特色方法为合成含有生物碱和药用化合物的吡咯提供了一种新颖的方法。
更新日期:2017-11-23
中文翻译:

N取代的吡咯-吡咯体系中Pd催化的区域选择性分子内脱氢C-5交叉偶联
通过N-取代的吡咯-唑的配体赋能的,Pd催化的,位点选择的,分子内的交叉偶联,直接合成了具有稠合的六元环和七元环的官能化多环吡咯-唑结构。在存在反应性C2-H的情况下C5-H的活化仍然是一个需要解决的挑战,并且有针对性地通过本方法解决该问题,具体方法是以83-100%的选择性生成环化产物。特色方法为合成含有生物碱和药用化合物的吡咯提供了一种新颖的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号