当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selectivity tuning over monometallic and bimetallic dehydrogenation catalysts: effects of support and particle size†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1039/c7cy01306j Konstantinos A. Goulas 1, 2, 3, 4, 5 , Yuying Song 1, 4, 6, 7 , Gregory R. Johnson 1, 2, 3, 4 , Justin P. Chen 1, 2, 3, 4, 8 , Amit A. Gokhale 1, 2, 3, 4, 9 , Lars C. Grabow 1, 4, 6, 7 , F. Dean Toste 2, 3, 4, 5, 8
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1039/c7cy01306j Konstantinos A. Goulas 1, 2, 3, 4, 5 , Yuying Song 1, 4, 6, 7 , Gregory R. Johnson 1, 2, 3, 4 , Justin P. Chen 1, 2, 3, 4, 8 , Amit A. Gokhale 1, 2, 3, 4, 9 , Lars C. Grabow 1, 4, 6, 7 , F. Dean Toste 2, 3, 4, 5, 8
Affiliation
|
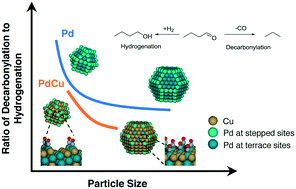
The efficacy of tandem dehydrogenation–condensation catalysts for the upgrade of bio-derived intermediates is largely determined by their relative (de-)hydrogenation and decarbonylation activity. Here, the effects of support and particle size of heterogeneous PdCu alloy catalysts on (de-)hydrogenation and decarbonlylation reactions were investigated using kinetic measurements, X-ray absorption spectroscopy and density functional theory (DFT). The chemical mismatch of Cu2+ with Ti4+ and Ca2+ prevents the substitution of Cu into the lattice of TiO2 or hydroxyapatite supports, and facilitates its alloying with Pd, resulting in improved selectivity for hydrogenation–dehydrogenation reactions compared to decarbonylation reactions. Based on kinetic measurements of butyraldehyde reactions over Pd and PdCu/SiO2 model catalysts, decarbonylation activity is attributed to the presence of Pd surface ensembles, while (de-)hydrogenation reactions are catalyzed by PdCu sites on the surface. This is consistent with selectivity and CO coverage trends with increasing conversion, and DFT-based microkinetic modeling. Selectivity control can also be achieved using the PdCu nanocluster size. Smaller nanoparticles favor the C–CO bond scission step of the decarbonylation reaction, due to the stronger binding of CO and alkyl species to sites of lower coordination. CO-induced segregation of reactive Pd atoms to under-coordinated step/edge sites also amplifies the geometric effect on the catalytic behavior.
中文翻译:

单金属和双金属脱氢催化剂的选择性调节:载体和粒径的影响†
串联脱氢-缩合催化剂对生物衍生中间体的提质很大程度上取决于它们的相对(脱)氢和脱羰活性。在这里,使用动力学测量,X射线吸收光谱和密度泛函理论(DFT)研究了载体和非均相PdCu合金催化剂的粒径对(脱氢)加氢和脱碳反应的影响。Cu 2+与Ti 4+和Ca 2+的化学错配可防止Cu取代成TiO 2的晶格或羟基磷灰石载体,并促进其与Pd合金化,与脱羰反应相比,提高了加氢-脱氢反应的选择性。基于在Pd和PdCu / SiO 2上丁醛反应的动力学测量在典型的催化剂中,脱羰活性归因于Pd表面团簇的存在,而(脱)氢化反应则是由表面PdCu位点催化的。这与随着转化率提高的选择性和一氧化碳覆盖率趋势以及基于DFT的微动力学建模相一致。使用PdCu纳米簇尺寸也可以实现选择性控制。较小的纳米粒子有利于脱羰反应的C–CO键断裂步骤,因为CO和烷基物种与较低配位的位点之间具有较强的结合力。CO诱导的反应性Pd原子偏析至配位不足的台阶/边缘位点也放大了对催化行为的几何影响。
更新日期:2017-11-22
中文翻译:

单金属和双金属脱氢催化剂的选择性调节:载体和粒径的影响†
串联脱氢-缩合催化剂对生物衍生中间体的提质很大程度上取决于它们的相对(脱)氢和脱羰活性。在这里,使用动力学测量,X射线吸收光谱和密度泛函理论(DFT)研究了载体和非均相PdCu合金催化剂的粒径对(脱氢)加氢和脱碳反应的影响。Cu 2+与Ti 4+和Ca 2+的化学错配可防止Cu取代成TiO 2的晶格或羟基磷灰石载体,并促进其与Pd合金化,与脱羰反应相比,提高了加氢-脱氢反应的选择性。基于在Pd和PdCu / SiO 2上丁醛反应的动力学测量在典型的催化剂中,脱羰活性归因于Pd表面团簇的存在,而(脱)氢化反应则是由表面PdCu位点催化的。这与随着转化率提高的选择性和一氧化碳覆盖率趋势以及基于DFT的微动力学建模相一致。使用PdCu纳米簇尺寸也可以实现选择性控制。较小的纳米粒子有利于脱羰反应的C–CO键断裂步骤,因为CO和烷基物种与较低配位的位点之间具有较强的结合力。CO诱导的反应性Pd原子偏析至配位不足的台阶/边缘位点也放大了对催化行为的几何影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号