当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrate-assisted photocatalytic efficiency of defective Eu-doped Pr(OH)3 nanostructures
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1039/c7cp06440c S. Aškrabić 1, 2, 3, 4, 5 , V. D. Araújo 6, 7, 8, 9 , M. Passacantando 10, 11, 12, 13, 14 , M. I. B. Bernardi 9, 15, 16, 17 , N. Tomić 1, 2, 3, 4, 5 , B. Dojčinović 3, 18, 19, 20, 21 , D. Manojlović 3, 22, 23, 24, 25 , B. Čalija 3, 25, 26, 27, 28 , M. Miletić 1, 2, 3, 4, 5 , Z. D. Dohčević-Mitrović 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1039/c7cp06440c S. Aškrabić 1, 2, 3, 4, 5 , V. D. Araújo 6, 7, 8, 9 , M. Passacantando 10, 11, 12, 13, 14 , M. I. B. Bernardi 9, 15, 16, 17 , N. Tomić 1, 2, 3, 4, 5 , B. Dojčinović 3, 18, 19, 20, 21 , D. Manojlović 3, 22, 23, 24, 25 , B. Čalija 3, 25, 26, 27, 28 , M. Miletić 1, 2, 3, 4, 5 , Z. D. Dohčević-Mitrović 1, 2, 3, 4, 5
Affiliation
|
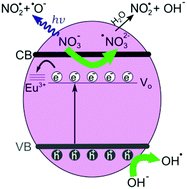
Pr(OH)3 one-dimensional nanostructures are a less studied member of lanthanide hydroxide nanostructures, which recently demonstrated an excellent adsorption capacity for organic pollutant removal from wastewater. In this study, Pr1−xEux(OH)3 (x = 0, 0.01, 0.03, and 0.05) defective nanostructures were synthesized by a facile and scalable microwave-assisted hydrothermal method using KOH as an alkaline metal precursor. The phase and surface composition, morphology, vibrational, electronic and optical properties of the as-prepared samples were characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), inductively coupled plasma optical emission spectrometry (ICP-OES), transmission electron microscopy (TEM), field emission scanning electron microscopy (FE-SEM), Raman, infrared (IR), photoluminescence (PL), and diffuse reflectance spectroscopy (DRS). It was deduced that the incorporation of Eu3+ ions promoted the formation of oxygen vacancies in the already defective Pr(OH)3, subsequently changing the Pr(OH)3 nanorod morphology. The presence of KNO3 phase was registered in the Eu-doped samples. The oxygen-deficient Eu-doped Pr(OH)3 nanostructures displayed an improved photocatalytic activity in the removal of reactive orange (RO16) dye under UV-vis light irradiation. An enhanced photocatalytic activity of the Eu-doped Pr(OH)3 nanostructures was caused by the synergetic effect of oxygen vacancies and Eu3+ (NO3−) ions present on the Pr(OH)3 surface, the charge separation efficiency and the formation of the reactive radicals. In addition, the 3% Eu-doped sample exhibited very good adsorptive properties due to different morphology and higher electrostatic attraction with the anionic dye. Pr1−xEux(OH)3 nanostructures with the possibility of tuning their adsorption/photocatalytic properties present a great potential for wastewater treatment.
中文翻译:

硝酸盐辅助的Eu掺杂Pr(OH)3缺陷纳米结构的光催化效率
Pr(OH)3一维纳米结构是氢氧化镧纳米结构研究较少的成员,最近证明了其对去除废水中有机污染物的出色吸附能力。在这项研究中,Pr 1− x Eu x(OH)3(x= 0、0.01、0.03和0.05)使用KOH作为碱金属前体,通过简便且可扩展的微波辅助水热法合成了有缺陷的纳米结构。通过X射线衍射(XRD),X射线光电子能谱(XPS),电感耦合等离子体发射光谱(ICP-OES)表征所制备样品的相和表面组成,形态,振动,电子和光学性质),透射电子显微镜(TEM),场发射扫描电子显微镜(FE-SEM),拉曼光谱,红外光谱(IR),光致发光(PL)和漫反射光谱(DRS)。可以推断,Eu 3+离子的引入促进了已经存在缺陷的Pr(OH)3中氧空位的形成。,随后改变Pr(OH)3纳米棒的形态。在Eu掺杂的样品中记录了KNO 3相的存在。缺氧的Eu掺杂Pr(OH)3纳米结构在紫外可见光照射下去除活性橙(RO16)染料方面表现出改善的光催化活性。的Eu掺杂镨(OH)的增强的光催化活性3个纳米结构是由氧空位和Eu的协同效应引起3+(NO 3 -离子存在于PR(OH))3表面,电荷分离效率和反应性自由基的形成。此外,由于阴离子的形态不同和较高的静电吸引性,掺杂3%Eu的样品表现出非常好的吸附性能。Pr 1- x Eu x(OH)3纳米结构具有调节其吸附/光催化性能的可能性,为废水处理提供了巨大的潜力。
更新日期:2017-11-23
中文翻译:

硝酸盐辅助的Eu掺杂Pr(OH)3缺陷纳米结构的光催化效率
Pr(OH)3一维纳米结构是氢氧化镧纳米结构研究较少的成员,最近证明了其对去除废水中有机污染物的出色吸附能力。在这项研究中,Pr 1− x Eu x(OH)3(x= 0、0.01、0.03和0.05)使用KOH作为碱金属前体,通过简便且可扩展的微波辅助水热法合成了有缺陷的纳米结构。通过X射线衍射(XRD),X射线光电子能谱(XPS),电感耦合等离子体发射光谱(ICP-OES)表征所制备样品的相和表面组成,形态,振动,电子和光学性质),透射电子显微镜(TEM),场发射扫描电子显微镜(FE-SEM),拉曼光谱,红外光谱(IR),光致发光(PL)和漫反射光谱(DRS)。可以推断,Eu 3+离子的引入促进了已经存在缺陷的Pr(OH)3中氧空位的形成。,随后改变Pr(OH)3纳米棒的形态。在Eu掺杂的样品中记录了KNO 3相的存在。缺氧的Eu掺杂Pr(OH)3纳米结构在紫外可见光照射下去除活性橙(RO16)染料方面表现出改善的光催化活性。的Eu掺杂镨(OH)的增强的光催化活性3个纳米结构是由氧空位和Eu的协同效应引起3+(NO 3 -离子存在于PR(OH))3表面,电荷分离效率和反应性自由基的形成。此外,由于阴离子的形态不同和较高的静电吸引性,掺杂3%Eu的样品表现出非常好的吸附性能。Pr 1- x Eu x(OH)3纳米结构具有调节其吸附/光催化性能的可能性,为废水处理提供了巨大的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号