当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative Proteomic Analysis of Lysine Acetylation in Trypanosomes
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1021/acs.jproteome.7b00603 Nilmar Silvio Moretti 1, 2 , Igor Cestari 2 , Atashi Anupama 2 , Ken Stuart 2 , Sergio Schenkman 1
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1021/acs.jproteome.7b00603 Nilmar Silvio Moretti 1, 2 , Igor Cestari 2 , Atashi Anupama 2 , Ken Stuart 2 , Sergio Schenkman 1
Affiliation
|
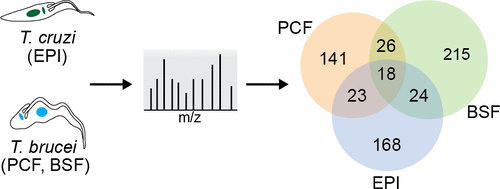
Protein acetylation is a post-translational modification regulating diverse cellular processes. By using proteomic approaches, we identified N-terminal and ε-lysine acetylated proteins in Trypanosoma cruzi and Trypanosoma brucei, which are protozoan parasites that cause significant human and animal diseases. We detected 288 lysine acetylation sites in 210 proteins of procyclic form, an insect stage of T. brucei, and 380 acetylation sites in 285 proteins in the form of the parasite that replicates in mammalian bloodstream. In T. cruzi insect proliferative form we found 389 ε-lysine-acetylated sites in 235 proteins. Notably, we found distinct acetylation profiles according to the developmental stage and species, with only 44 common proteins between T. brucei stages and 18 in common between the two species. While K-ac proteins from T. cruzi are enriched in enzymes involved in oxidation/reduction balance, required for the parasite survival in the host, in T. brucei, most K-ac proteins are enriched in metabolic processes, essential for its adaptation in its hosts. We also identified in both parasites a quite variable N-terminal acetylation sites. Our results suggest that protein acetylation is involved in differential regulation of multiple cellular processes in Trypanosomes, contributing to our understanding of the essential mechanisms for parasite infection and survival.
中文翻译:

锥虫赖氨酸乙酰化的比较蛋白质组学分析
蛋白质乙酰化是一种调节多种细胞过程的翻译后修饰。通过使用蛋白质组学方法,我们在克氏锥虫和布氏锥虫中鉴定了 N-末端和 ε-赖氨酸乙酰化蛋白,它们是导致重大人类和动物疾病的原生动物寄生虫。我们在T. brucei的昆虫阶段的 210 种前循环形式蛋白质中检测到 288 个赖氨酸乙酰化位点,在哺乳动物血液中复制的寄生虫形式的 285 种蛋白质中检测到 380 个乙酰化位点。在T. cruzi在昆虫增殖形式中,我们在 235 种蛋白质中发现了 389 个 ε-赖氨酸乙酰化位点。值得注意的是,我们根据发育阶段和物种发现了不同的乙酰化谱,T. brucei阶段之间只有 44 种常见蛋白质,两种物种之间共有 18 种。虽然来自T. cruzi的 K-ac 蛋白富含参与氧化/还原平衡的酶,这是寄生虫在宿主中存活所必需的,但在T. brucei, 大多数 K-ac 蛋白都富含代谢过程,这对于它在宿主中的适应至关重要。我们还在两种寄生虫中发现了一个非常可变的 N 末端乙酰化位点。我们的结果表明,蛋白质乙酰化参与了锥虫中多个细胞过程的差异调节,有助于我们了解寄生虫感染和存活的基本机制。
更新日期:2017-12-11
中文翻译:

锥虫赖氨酸乙酰化的比较蛋白质组学分析
蛋白质乙酰化是一种调节多种细胞过程的翻译后修饰。通过使用蛋白质组学方法,我们在克氏锥虫和布氏锥虫中鉴定了 N-末端和 ε-赖氨酸乙酰化蛋白,它们是导致重大人类和动物疾病的原生动物寄生虫。我们在T. brucei的昆虫阶段的 210 种前循环形式蛋白质中检测到 288 个赖氨酸乙酰化位点,在哺乳动物血液中复制的寄生虫形式的 285 种蛋白质中检测到 380 个乙酰化位点。在T. cruzi在昆虫增殖形式中,我们在 235 种蛋白质中发现了 389 个 ε-赖氨酸乙酰化位点。值得注意的是,我们根据发育阶段和物种发现了不同的乙酰化谱,T. brucei阶段之间只有 44 种常见蛋白质,两种物种之间共有 18 种。虽然来自T. cruzi的 K-ac 蛋白富含参与氧化/还原平衡的酶,这是寄生虫在宿主中存活所必需的,但在T. brucei, 大多数 K-ac 蛋白都富含代谢过程,这对于它在宿主中的适应至关重要。我们还在两种寄生虫中发现了一个非常可变的 N 末端乙酰化位点。我们的结果表明,蛋白质乙酰化参与了锥虫中多个细胞过程的差异调节,有助于我们了解寄生虫感染和存活的基本机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号