当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase equilibrium in the System KH2PO4 + KCl + H3PO4 at 298.15 K and 308.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.jced.7b00554 Sheng-qiang Lin 1 , Jian-hua Tang 1 , Jian Teng 1 , Debiao Liu 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.jced.7b00554 Sheng-qiang Lin 1 , Jian-hua Tang 1 , Jian Teng 1 , Debiao Liu 1
Affiliation
|
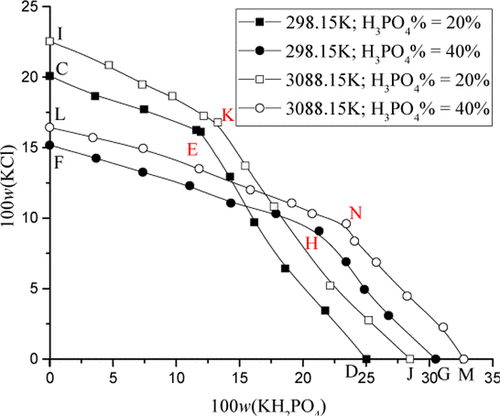
The solubility of (KH2PO4 + KCl + H3PO4) at 298.15 K and 308.15 K were determined, using isothermal solution saturation. The equilibrium phase solid was verified by X-ray diffraction (XRD). On the basis of the experimental data, the phase diagrams were plotted. The crystalline regions of KH2PO4 and KCl were determined. The phase equilibrium in different phosphoric acid concentrations are compared and discussed. This comparison further illustrates that the phosphoric acid concentration can influence the equilibrium besides temperatures. All results can offer a fundamental basis for crystallization and separation processes.
中文翻译:

系统KH 2 PO 4 + KCl + H 3 PO 4在298.15 K和308.15 K时的相平衡
使用等温溶液饱和度,确定(KH 2 PO 4 + KCl + H 3 PO 4)在298.15 K和308.15 K的溶解度。平衡相固体通过X射线衍射(XRD)验证。根据实验数据,绘制了相图。确定了KH 2 PO 4和KCl的结晶区域。比较和讨论了不同磷酸浓度下的相平衡。该比较进一步说明了磷酸浓度除了影响温度外还可以影响平衡。所有结果均可为结晶和分离过程提供基础。
更新日期:2017-11-23
中文翻译:

系统KH 2 PO 4 + KCl + H 3 PO 4在298.15 K和308.15 K时的相平衡
使用等温溶液饱和度,确定(KH 2 PO 4 + KCl + H 3 PO 4)在298.15 K和308.15 K的溶解度。平衡相固体通过X射线衍射(XRD)验证。根据实验数据,绘制了相图。确定了KH 2 PO 4和KCl的结晶区域。比较和讨论了不同磷酸浓度下的相平衡。该比较进一步说明了磷酸浓度除了影响温度外还可以影响平衡。所有结果均可为结晶和分离过程提供基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号