当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption Equilibrium, Kinetics, and Thermodynamic Studies of Cefpirome Sulfate by Using Macroporous Resin
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.jced.7b00629 Feng Xue 1 , Fugang Wang 1 , Shuai Chen 1 , Shengui Ju 1 , Weihong Xing 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.jced.7b00629 Feng Xue 1 , Fugang Wang 1 , Shuai Chen 1 , Shengui Ju 1 , Weihong Xing 1
Affiliation
|
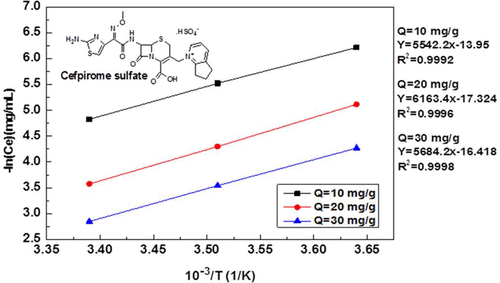
The adsorption thermodynamics, kinetics, and isotherm parameters of cefpirome sulfate in aqueous solution on macroporous resin (XAD-16) were studied. Using static equilibrium tests, the fitting of resin adsorption data were calculated by the isothermal adsorption model. The fitting results show that Freundlich equation can adequately fit the adsorption isotherm. Meanwhile, the derived adsorption constants and their temperature dependencies from Freundlich isotherm had been used to calculate the corresponding thermodynamic quantities, such as the free energy of adsorption, heat, and entropy of adsorption. The thermodynamic data indicated that XAD-16 resin adsorption of cefpirome sulfate in aqueous solution was a spontaneous exothermic process, which was characterized by physical adsorption. The influences of initial concentration, bed height, and residence time on the breakthrough curve were examined by dynamic tests and the optimal parameters were defined.
中文翻译:

大孔树脂对硫酸头孢匹罗的吸附平衡,动力学和热力学研究
研究了大孔树脂(XAD-16)上硫酸头孢吡罗酮在水溶液中的吸附热力学,动力学和等温线参数。使用静态平衡测试,通过等温吸附模型计算树脂吸附数据的拟合。拟合结果表明,Freundlich方程可以充分拟合吸附等温线。同时,利用Freundlich等温线推导的吸附常数及其温度依赖性,计算了相应的热力学量,如吸附的自由能,热量和吸附熵。热力学数据表明,XAD-16树脂在水溶液中吸附硫酸头孢吡肟是自发的放热过程,其特征在于物理吸附。初始浓度,床高,
更新日期:2017-11-23
中文翻译:

大孔树脂对硫酸头孢匹罗的吸附平衡,动力学和热力学研究
研究了大孔树脂(XAD-16)上硫酸头孢吡罗酮在水溶液中的吸附热力学,动力学和等温线参数。使用静态平衡测试,通过等温吸附模型计算树脂吸附数据的拟合。拟合结果表明,Freundlich方程可以充分拟合吸附等温线。同时,利用Freundlich等温线推导的吸附常数及其温度依赖性,计算了相应的热力学量,如吸附的自由能,热量和吸附熵。热力学数据表明,XAD-16树脂在水溶液中吸附硫酸头孢吡肟是自发的放热过程,其特征在于物理吸附。初始浓度,床高,











































 京公网安备 11010802027423号
京公网安备 11010802027423号