当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Tunable and Enantioselective Hetero-Diels–Alder Reaction Provides Access to Distinct Piperidinoyl Spirooxindoles
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-11-22 04:17:59 , DOI: 10.1002/anie.201708355 Samydurai Jayakumar 1 , Kathrin Louven 2 , Carsten Strohmann 2 , Kamal Kumar 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-11-22 04:17:59 , DOI: 10.1002/anie.201708355 Samydurai Jayakumar 1 , Kathrin Louven 2 , Carsten Strohmann 2 , Kamal Kumar 1
Affiliation
|
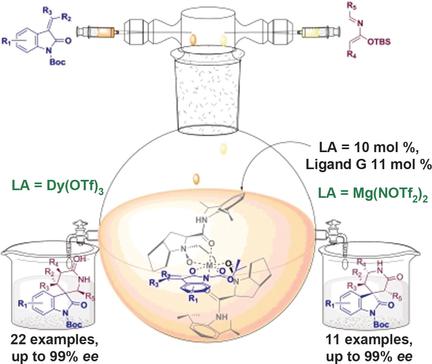
Tuned to ring! DyIII and MgII salts with an N,N′-dioxide ligand catalyze enantioselective hetero-Diels–Alder reactions of silyloxy-2-azadienes with alkylidene oxindoles to selectively form 3,3′- and 3,4′-piperidinoyl-spirooxindoles, respectively, in excellent yields with excellent enantiomeric ratios. Boc=tert-butoxycarbonyl, TBS=tert-butyldimethylsilyl, Tf=trifluoromethanesulfonyl.
中文翻译:

可调谐和对映选择性的杂-Diels-Alder反应可提供不同的哌啶子基螺并氧杂吲哚
调好响了!带有N,N'-二氧化物配体的Dy III和Mg II盐催化甲硅烷氧基-2-氮杂二烯与亚烷基ide吲哚的对映选择性杂狄尔斯-阿尔德反应,选择性地形成3,3'-和3,4'-哌啶子基-螺氧并吲哚分别以优异的收率和优异的对映体比率获得。Boc =叔丁氧羰基,TBS =叔丁基二甲基甲硅烷基,Tf =三氟甲磺酰基。
更新日期:2017-11-22
中文翻译:

可调谐和对映选择性的杂-Diels-Alder反应可提供不同的哌啶子基螺并氧杂吲哚
调好响了!带有N,N'-二氧化物配体的Dy III和Mg II盐催化甲硅烷氧基-2-氮杂二烯与亚烷基ide吲哚的对映选择性杂狄尔斯-阿尔德反应,选择性地形成3,3'-和3,4'-哌啶子基-螺氧并吲哚分别以优异的收率和优异的对映体比率获得。Boc =叔丁氧羰基,TBS =叔丁基二甲基甲硅烷基,Tf =三氟甲磺酰基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号