当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Albumin/sulfonamide stabilized iron porphyrin metal organic framework nanocomposites: targeting tumor hypoxia by carbonic anhydrase IX inhibition and T1–T2 dual mode MRI guided photodynamic/photothermal therapy†
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1039/c7tb02818k Wei Zhu 1, 2, 3, 4 , Yao Liu 3, 4, 5, 6, 7 , Zhe Yang 1, 2, 3, 4 , Li Zhang 1, 2, 3, 4 , Liji Xiao 1, 2, 3, 4 , Pei Liu 1, 2, 3, 4 , Jing Wang 1, 2, 3, 4 , Changfeng Yi 1, 2, 3, 4 , Zushun Xu 1, 2, 3, 4 , Jinghua Ren 3, 4, 5, 6, 7
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1039/c7tb02818k Wei Zhu 1, 2, 3, 4 , Yao Liu 3, 4, 5, 6, 7 , Zhe Yang 1, 2, 3, 4 , Li Zhang 1, 2, 3, 4 , Liji Xiao 1, 2, 3, 4 , Pei Liu 1, 2, 3, 4 , Jing Wang 1, 2, 3, 4 , Changfeng Yi 1, 2, 3, 4 , Zushun Xu 1, 2, 3, 4 , Jinghua Ren 3, 4, 5, 6, 7
Affiliation
|
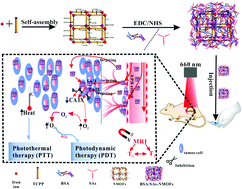
Exploring a nanotheranostic agent with image guided highly efficient therapeutic properties is greatly significant for tumor screening and treatment. Herein, we construct a novel nanoplatform, composed of low cost bovine serum albumin (BSA), sulfonamides (SAs) and iron porphyrin nanoscale metal organic frameworks (NMOFs), which possesses the capability of active targeting to tumor cells and magnetic resonance imaging (MRI) can be used to guide synergetic photodynamic/photothermal therapy. These constructed BSA/SAs–NMOF nanoplatforms can be accumulated more at tumor sites due to modification of the nanoparticles with BSA/SA complexes, allowing this system to achieve long circulation in vivo and to actively target to carbonic anhydrase (CA) IX of tumor cells by the SAs. Moreover, the magnetic iron ion and porphyrin serve as the metal centre and organic ligand of novel NMOFs which exhibit a T1–T2 weighted MRI effect (r1 = 2.7 mM−1 s−1 and r2 = 19.68 mM−1 s−1) and allow synergetic photodynamic/photothermal therapy of tumors. In vitro reactive oxygen species (ROS) detection and photothermal temperature change results revealed that these BSA/SAs–NMOF nanoplatforms could exhibit a great PDT effect in tumor cells, even under hypoxic conditions, and a remarkable PTT effect with a photothermal conversion efficiency of 40.53%. What's more, there was a greater fatality rate of 4T1 cancer cells in the single wavelength induced PDT & PTT group (95%) than in the PDT or PTT monotherapy groups (nearly 80%), and the growth of a solid tumor was more effectively inhibited by PDT & PTT than by single PTT or PDT. This work provides a novel nanoplatform for targeting tumor hypoxia and achieved highly efficient treatment of tumors based on PDT and PTT.
中文翻译:

白蛋白/磺酰胺稳定的铁卟啉金属有机骨架纳米复合材料:通过碳酸酐酶IX抑制和T 1 - T 2双模MRI引导的光动力/光热疗法来靶向肿瘤缺氧†
探索具有图像指导的高效治疗特性的纳米治疗剂对肿瘤的筛查和治疗具有重大意义。本文中,我们构建了一种新型的纳米平台,该平台由低成本的牛血清白蛋白(BSA),磺酰胺(SAs)和铁卟啉纳米级金属有机骨架(NMOF)组成,具有主动靶向肿瘤细胞和磁共振成像(MRI)的能力。 )可用于指导协同光动力/光热疗法。这些构造的BSA / SAs–NMOF纳米平台可以通过在BSA / SA复合物中修饰纳米颗粒而在肿瘤部位积累更多,从而使该系统能够在体内实现长循环并通过SA主动靶向肿瘤细胞的碳酸酐酶(CA)IX。此外,磁性铁离子和卟啉是新型NMOF的金属中心和有机配体,它们表现出T 1 – T 2加权MRI效应(r 1 = 2.7 mM -1 s -1和r 2 = 19.68 mM -1 s -1)并允许对肿瘤进行协同光动力/光热疗法。体外活性氧(ROS)检测和光热温度变化结果表明,即使在低氧条件下,这些BSA / SAs–NMOF纳米平台也可能在肿瘤细胞中表现出极大的PDT效应,并具有显着的PTT效应,光热转换效率为40.53%。而且,单波长诱导的PDT和PTT组(95%)中的4T1癌细胞的死亡率高于PDT或PTT单药治疗组(近80%),并且实体瘤的生长更有效与单个PTT或PDT相比,受PDT和PTT抑制。这项工作提供了一种新型的靶向肿瘤缺氧的纳米平台,并基于PDT和PTT实现了对肿瘤的高效治疗。
更新日期:2017-11-22
中文翻译:

白蛋白/磺酰胺稳定的铁卟啉金属有机骨架纳米复合材料:通过碳酸酐酶IX抑制和T 1 - T 2双模MRI引导的光动力/光热疗法来靶向肿瘤缺氧†
探索具有图像指导的高效治疗特性的纳米治疗剂对肿瘤的筛查和治疗具有重大意义。本文中,我们构建了一种新型的纳米平台,该平台由低成本的牛血清白蛋白(BSA),磺酰胺(SAs)和铁卟啉纳米级金属有机骨架(NMOF)组成,具有主动靶向肿瘤细胞和磁共振成像(MRI)的能力。 )可用于指导协同光动力/光热疗法。这些构造的BSA / SAs–NMOF纳米平台可以通过在BSA / SA复合物中修饰纳米颗粒而在肿瘤部位积累更多,从而使该系统能够在体内实现长循环并通过SA主动靶向肿瘤细胞的碳酸酐酶(CA)IX。此外,磁性铁离子和卟啉是新型NMOF的金属中心和有机配体,它们表现出T 1 – T 2加权MRI效应(r 1 = 2.7 mM -1 s -1和r 2 = 19.68 mM -1 s -1)并允许对肿瘤进行协同光动力/光热疗法。体外活性氧(ROS)检测和光热温度变化结果表明,即使在低氧条件下,这些BSA / SAs–NMOF纳米平台也可能在肿瘤细胞中表现出极大的PDT效应,并具有显着的PTT效应,光热转换效率为40.53%。而且,单波长诱导的PDT和PTT组(95%)中的4T1癌细胞的死亡率高于PDT或PTT单药治疗组(近80%),并且实体瘤的生长更有效与单个PTT或PDT相比,受PDT和PTT抑制。这项工作提供了一种新型的靶向肿瘤缺氧的纳米平台,并基于PDT和PTT实现了对肿瘤的高效治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号