当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electronic structure and dynamics of torsion-locked photoactive yellow protein chromophores
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1039/c7cp06950b Alice Henley 1, 2, 3, 4 , Matus E. Diveky 1, 2, 3, 4 , Anand M. Patel 1, 2, 3, 4 , Michael A. Parkes 1, 2, 3, 4 , James C. Anderson 1, 2, 3, 4 , Helen H. Fielding 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1039/c7cp06950b Alice Henley 1, 2, 3, 4 , Matus E. Diveky 1, 2, 3, 4 , Anand M. Patel 1, 2, 3, 4 , Michael A. Parkes 1, 2, 3, 4 , James C. Anderson 1, 2, 3, 4 , Helen H. Fielding 1, 2, 3, 4
Affiliation
|
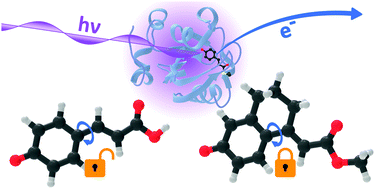
The photocycle of photoactive yellow protein (PYP) begins with small-scale torsional motions of the chromophore leading to large-scale movements of the protein scaffold triggering a biological response. The role of single-bond torsional molecular motions of the chromophore in the initial steps of the PYP photocycle are not fully understood. Here, we employ anion photoelectron spectroscopy measurements and quantum chemistry calculations to investigate the electronic relaxation dynamics following photoexcitation of four model chromophores, para-coumaric acid, its methyl ester, and two analogues with aliphatic bridges hindering torsional motions around the single bonds adjacent to the alkene group. Following direct photoexcitation of S1 at 400 nm, we find that both single bond rotations play a role in steering the PYP chromophore through the S1/S0 conical intersection but that rotation around the single bond between the alkene moiety and the phenoxide group is particularly important. Following photoexcitation of higher lying electronic states in the range 346–310 nm, we find that rotation around the single bond between the alkene and phenoxide groups also plays a key role in the electronic relaxation from higher lying states to the S1 state. These results have potential applications in tuning the photoresponse of photoactive proteins and materials with chromophores based on PYP.
中文翻译:

扭转锁定的光敏黄色蛋白发色团的电子结构和动力学
光活性黄色蛋白质(PYP)的光循环以发色团的小规模扭转运动开始,导致蛋白质支架的大规模运动触发生物反应。在PYP光循环的初始步骤中,生色团的单键扭转分子运动的作用尚不完全清楚。在这里,我们采用阴离子光电子能谱测量和量子化学计算来研究光激发四个模型生色团,对香豆酸,其甲酯和两个类似的具有脂肪族桥的类似物,它们阻碍了与该键相邻的单键周围的扭转运动。烯基。在直接光激发S 1之后在400 nm处,我们发现两个单键旋转都在控制PYP生色团通过S 1 / S 0圆锥形交点中起作用,但是围绕烯烃部分和酚盐基团之间的单键旋转特别重要。在346-310 nm范围内对较高电子态进行光激发后,我们发现围绕烯烃和酚盐基团之间单键的旋转在从高电子态到S 1态的电子弛豫中也起着关键作用。这些结果在利用基于PYP的生色团调节光敏蛋白和材料的光响应方面具有潜在的应用价值。
更新日期:2017-11-22
中文翻译:

扭转锁定的光敏黄色蛋白发色团的电子结构和动力学
光活性黄色蛋白质(PYP)的光循环以发色团的小规模扭转运动开始,导致蛋白质支架的大规模运动触发生物反应。在PYP光循环的初始步骤中,生色团的单键扭转分子运动的作用尚不完全清楚。在这里,我们采用阴离子光电子能谱测量和量子化学计算来研究光激发四个模型生色团,对香豆酸,其甲酯和两个类似的具有脂肪族桥的类似物,它们阻碍了与该键相邻的单键周围的扭转运动。烯基。在直接光激发S 1之后在400 nm处,我们发现两个单键旋转都在控制PYP生色团通过S 1 / S 0圆锥形交点中起作用,但是围绕烯烃部分和酚盐基团之间的单键旋转特别重要。在346-310 nm范围内对较高电子态进行光激发后,我们发现围绕烯烃和酚盐基团之间单键的旋转在从高电子态到S 1态的电子弛豫中也起着关键作用。这些结果在利用基于PYP的生色团调节光敏蛋白和材料的光响应方面具有潜在的应用价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号