当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of spherical ice-shells inside carbon fullerenes
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7cp05987f Roxanne M. Tutchton 1, 2, 3, 4 , Zhigang Wu 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7cp05987f Roxanne M. Tutchton 1, 2, 3, 4 , Zhigang Wu 1, 2, 3, 4
Affiliation
|
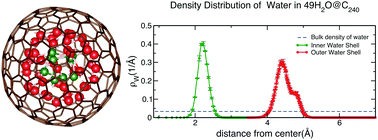
The structural and dynamic properties of encapsulated water inside fullerene cages, C60 to C320, were investigated employing classical molecular dynamics simulations. We find that the confined water forms single to multiple concentric, spherical shells as the size of the fullerene increases. This is possible due to the reduced number of hydrogen bonds per water molecule in the nanoscale liquid as compared to bulk water, allowing the encapsulated H2O molecules to imitate the shape of the confining boundary. These water-cluster shells exhibit solid-like behavior at temperatures as high as 500 K. Our current findings complement the existing literature on water confined by sp2-hybridized nanocarbon structures including one dimensional nanotubes and two dimensional graphene sheets.
中文翻译:

碳富勒烯内部形成球形冰壳
使用经典的分子动力学模拟研究了富勒烯笼中C 60至C 320内包封水的结构和动力学性质。我们发现,随着富勒烯尺寸的增加,承压水形成单个或多个同心的球形壳。这是可能的,因为与大量水相比,纳米级液体中每个水分子的氢键数目减少,从而使封装的H 2 O分子模仿边界边界的形状。这些水簇壳在高达500 K的温度下表现出类似固体的行为。我们的发现补充了关于sp 2限制的水的现有文献。-杂交的纳米碳结构,包括一维纳米管和二维石墨烯片。
更新日期:2017-11-22
中文翻译:

碳富勒烯内部形成球形冰壳
使用经典的分子动力学模拟研究了富勒烯笼中C 60至C 320内包封水的结构和动力学性质。我们发现,随着富勒烯尺寸的增加,承压水形成单个或多个同心的球形壳。这是可能的,因为与大量水相比,纳米级液体中每个水分子的氢键数目减少,从而使封装的H 2 O分子模仿边界边界的形状。这些水簇壳在高达500 K的温度下表现出类似固体的行为。我们的发现补充了关于sp 2限制的水的现有文献。-杂交的纳米碳结构,包括一维纳米管和二维石墨烯片。











































 京公网安备 11010802027423号
京公网安备 11010802027423号