当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydration of iron–porphyrins: ab initio quantum mechanical charge field molecular dynamics simulation study
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1039/c7cp04436d Syed Tarique Moin 1, 2, 3 , Thomas S. Hofer 4, 5, 6, 7
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1039/c7cp04436d Syed Tarique Moin 1, 2, 3 , Thomas S. Hofer 4, 5, 6, 7
Affiliation
|
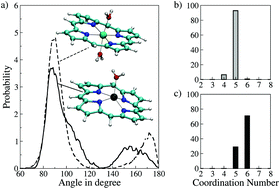
The ab initio quantum mechanical charge field molecular dynamics (QMCF-MD) simulation approach was successfully applied to Fe2+–P and Fe3+–P in water to evaluate their structural, dynamical and energetic properties. Based on the structural data, it was found that Fe2+–P accommodates one water molecule in the first coordination sphere of the Fe2+ ion including the four nitrogen atoms of the porphyrin system coordinating with central metal species. On the other hand, two water molecules were coordinated to Fe3+–P, thus forming a hexa-coordinated species. Comparison of dynamical properties such as the vibrational power spectrum and ligand mean residence times to other metal-free porphyrin systems demonstrate the ions' influence on the hydration structure, enabling a characterisation of the strong interaction of the ions which greatly reduces the hydrogen bonding potential of the complex. The association of water molecules with the metal ions in both solutes was quantified by computing the free energy of binding obtained via the potential of mean force. This further confirmed the strong association of water to the metal ions which was conversely weak as inferred from the energetic data for the Fe2+–P system.
中文翻译:

铁卟啉的水合:从头算量子力学电荷场分子动力学模拟研究
在从头量子力学电荷场分子动力学(QMCF-MD)模拟方法被成功地应用于成Fe 2+ -P和Fe 3+ -P在水中以评价它们的结构,动力和能量性质。根据结构数据,发现Fe 2+ -P在Fe 2+离子的第一个配位域中容纳一个水分子,其中包括与中心金属物种配位的卟啉系统的四个氮原子。另一方面,两个水分子与Fe 3+配位-P,从而形成六配位物种。比较动力学性质(例如振动能谱和配体对其他无金属卟啉系统的平均停留时间)可证明离子对水合结构的影响,从而表征了离子的强相互作用,从而大大降低了氢键的氢键势。复杂。通过计算通过平均力势获得的结合自由能,可以定量两种溶质中水分子与金属离子的缔合。这进一步证实了水与金属离子的强缔合,而从Fe 2+ -P体系的高能数据推断,这种缔合反而较弱。
更新日期:2017-11-22
中文翻译:

铁卟啉的水合:从头算量子力学电荷场分子动力学模拟研究
在从头量子力学电荷场分子动力学(QMCF-MD)模拟方法被成功地应用于成Fe 2+ -P和Fe 3+ -P在水中以评价它们的结构,动力和能量性质。根据结构数据,发现Fe 2+ -P在Fe 2+离子的第一个配位域中容纳一个水分子,其中包括与中心金属物种配位的卟啉系统的四个氮原子。另一方面,两个水分子与Fe 3+配位-P,从而形成六配位物种。比较动力学性质(例如振动能谱和配体对其他无金属卟啉系统的平均停留时间)可证明离子对水合结构的影响,从而表征了离子的强相互作用,从而大大降低了氢键的氢键势。复杂。通过计算通过平均力势获得的结合自由能,可以定量两种溶质中水分子与金属离子的缔合。这进一步证实了水与金属离子的强缔合,而从Fe 2+ -P体系的高能数据推断,这种缔合反而较弱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号