PLOS Medicine ( IF 10.5 ) Pub Date : 2017-11-21 , DOI: 10.1371/journal.pmed.1002446 Lorna Dunning 1, 2 , Jordan A Francke 2 , Divya Mallampati 3 , Rachel L MacLean 2 , Martina Penazzato 4 , Taige Hou 2 , Landon Myer 1, 5 , Elaine J Abrams 6, 7 , Rochelle P Walensky 2, 8, 9, 10 , Valériane Leroy 11 , Kenneth A Freedberg 2, 8, 10, 12 , Andrea Ciaranello 2, 8
|
Background
The specificity of nucleic acid amplification tests (NAATs) used for early infant diagnosis (EID) of HIV infection is <100%, leading some HIV-uninfected infants to be incorrectly identified as HIV-infected. The World Health Organization recommends that infants undergo a second NAAT to confirm any positive test result, but implementation is limited. Our objective was to determine the impact and cost-effectiveness of confirmatory HIV testing for EID programmes in South Africa.
Method and findings
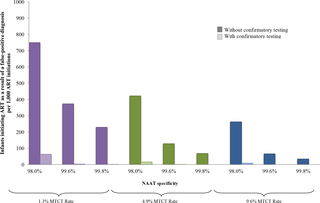
Using the Cost-effectiveness of Preventing AIDS Complications (CEPAC)–Pediatric model, we simulated EID testing at age 6 weeks for HIV-exposed infants without and with confirmatory testing. We assumed a NAAT cost of US$25, NAAT specificity of 99.6%, NAAT sensitivity of 100% for infants infected in pregnancy or at least 4 weeks prior to testing, and a mother-to-child transmission (MTCT) rate at 12 months of 4.9%; we simulated guideline-concordant rates of testing uptake, result return, and antiretroviral therapy (ART) initiation (100%). After diagnosis, infants were linked to and retained in care for 10 years (false-positive) or lifelong (true-positive). All parameters were varied widely in sensitivity analyses. Outcomes included number of infants with false-positive diagnoses linked to ART per 1,000 ART initiations, life expectancy (LE, in years) and per-person lifetime HIV-related healthcare costs. Both without and with confirmatory testing, LE was 26.2 years for HIV-infected infants and 61.4 years for all HIV-exposed infants; clinical outcomes for truly infected infants did not differ by strategy. Without confirmatory testing, 128/1,000 ART initiations were false-positive diagnoses; with confirmatory testing, 1/1,000 ART initiations were false-positive diagnoses. Because confirmatory testing averted costly HIV care and ART in truly HIV-uninfected infants, it was cost-saving: total cost US$1,790/infant tested, compared to US$1,830/infant tested without confirmatory testing. Confirmatory testing remained cost-saving unless NAAT cost exceeded US$400 or the HIV-uninfected status of infants incorrectly identified as infected was ascertained and ART stopped within 3 months of starting. Limitations include uncertainty in the data used in the model, which we examined with sensitivity and uncertainty analyses. We also excluded clinical harms to HIV-uninfected infants incorrectly treated with ART after false-positive diagnosis (e.g., medication toxicities); including these outcomes would further increase the value of confirmatory testing.
Conclusions
Without confirmatory testing, in settings with MTCT rates similar to that of South Africa, more than 10% of infants who initiate ART may reflect false-positive diagnoses. Confirmatory testing prevents inappropriate HIV diagnosis, is cost-saving, and should be adopted in all EID programmes.
中文翻译:

南非早期婴儿艾滋病毒诊断项目中验证性检测的价值:成本效益分析
背景
用于婴儿早期艾滋病毒感染诊断(EID)的核酸扩增试验(NAAT)的特异性<100%,导致一些未感染艾滋病毒的婴儿被错误地识别为艾滋病毒感染者。世界卫生组织建议婴儿接受第二次 NAAT 以确认任何阳性检测结果,但实施情况有限。我们的目标是确定南非 EID 项目的艾滋病毒确证检测的影响和成本效益。
方法和结果
使用预防艾滋病并发症的成本效益(CEPAC)-儿科模型,我们模拟了在没有和有验证性检测的情况下对 HIV 暴露婴儿 6 周龄时的 EID 检测。我们假设 NAAT 成本为 25 美元,NAAT 特异性为 99.6%,怀孕期间或检测前至少 4 周感染的婴儿的 NAAT 敏感性为 100%,母婴传播 (MTCT) 率为 12 个月。 4.9%;我们模拟了检测采用率、结果返回率和抗逆转录病毒治疗 (ART) 启动率 (100%) 的指南一致率。诊断后,婴儿将被联系并保留 10 年(假阳性)或终身(真阳性)。所有参数在敏感性分析中变化很大。结果包括每 1,000 次 ART 治疗中出现与 ART 相关的假阳性诊断的婴儿数量、预期寿命(LE,以年为单位)以及人均终生 HIV 相关医疗费用。无论是否进行验证性检测,感染 HIV 的婴儿的 LE 为 26.2 岁,所有暴露于 HIV 的婴儿的 LE 为 61.4 岁;真正感染的婴儿的临床结果并没有因策略而异。如果没有进行确认性检测,128/1,000 的 ART 启动是假阳性诊断;通过验证性检测,1/1,000 的 ART 启动是假阳性诊断。由于确认性检测避免了对真正未感染艾滋病毒的婴儿进行昂贵的艾滋病毒护理和抗逆转录病毒治疗,因此可以节省成本:每个接受检测的婴儿的总成本为 1,790 美元,而没有进行确认性检测的每个婴儿的总成本为 1,830 美元。除非 NAAT 费用超过 400 美元,或者确定了被错误地识别为感染的婴儿的 HIV 未感染状态,并且 ART 在开始后 3 个月内停止,否则确认性检测仍然可以节省成本。 局限性包括模型中使用的数据的不确定性,我们通过敏感性和不确定性分析进行了检查。我们还排除了在假阳性诊断后未正确接受 ART 治疗的未感染 HIV 婴儿的临床伤害(例如药物毒性);包括这些结果将进一步增加验证性测试的价值。
结论
如果没有确证性检测,在母婴传播率与南非相似的地区,超过 10% 的接受 ART 的婴儿可能会出现假阳性诊断。确认性检测可以防止不适当的艾滋病毒诊断,可以节省成本,应该在所有 EID 项目中采用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号