当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron‐Sulfate‐Catalyzed Direct Dehydrogenative Coupling Cyanation of Secondary Phenylamines
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-12-12 , DOI: 10.1002/ajoc.201700524 Mingming Huang 1 , Qingfu Deng 1 , Qin Gao 1 , Jian Shi 2 , Xiaohui Zhang 1 , Yan Xiong 1, 3
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-12-12 , DOI: 10.1002/ajoc.201700524 Mingming Huang 1 , Qingfu Deng 1 , Qin Gao 1 , Jian Shi 2 , Xiaohui Zhang 1 , Yan Xiong 1, 3
Affiliation
|
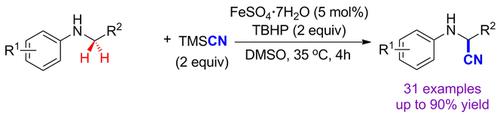
A direct dehydrogenative coupling cyanation of secondary phenylamines was developed under iron sulfate catalysis by utilizing trimethylsilyl cyanide as a cyano source and tert‐butyl hydroperoxide as oxidant. A variety of α‐aminonitriles, which commonly serve as versatile synthetically important intermediates, were synthesized in isolated yields of up to 90 %. Based on control experiments, the cyanation mechanism might involve a single‐electron transfer and a subsequent formation of an iminium ion.
中文翻译:

硫酸铁催化仲苯胺的直接脱氢偶联氰化
通过使用三甲基甲硅烷基氰化物作为氰基源和叔丁基氢过氧化物作为氧化剂,在硫酸铁催化下开发了仲苯胺的直接脱氢偶合氰化反应。合成了通常用作通用的重要合成中间体的多种α-氨基腈,分离产率高达90%。根据控制实验,氰化机理可能涉及单电子转移和随后形成亚胺离子。
更新日期:2017-12-12
中文翻译:

硫酸铁催化仲苯胺的直接脱氢偶联氰化
通过使用三甲基甲硅烷基氰化物作为氰基源和叔丁基氢过氧化物作为氧化剂,在硫酸铁催化下开发了仲苯胺的直接脱氢偶合氰化反应。合成了通常用作通用的重要合成中间体的多种α-氨基腈,分离产率高达90%。根据控制实验,氰化机理可能涉及单电子转移和随后形成亚胺离子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号