当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Chiral Acyclic Pyrimidine Nucleosides with a Sulfur-Containing Side Chain via Enantioselective Tandem Conjugate Addition/Protonation
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-22 03:00:29 , DOI: 10.1002/ajoc.201700533 Jian-Ping Li 1 , Hao-Ran Tuo 1 , Ming-Sheng Xie 1 , Bo Kang 1 , Gui-Rong Qu 1 , Hai-Ming Guo 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-22 03:00:29 , DOI: 10.1002/ajoc.201700533 Jian-Ping Li 1 , Hao-Ran Tuo 1 , Ming-Sheng Xie 1 , Bo Kang 1 , Gui-Rong Qu 1 , Hai-Ming Guo 1
Affiliation
|
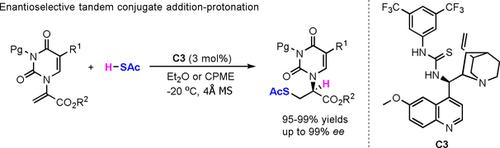
AdPro: A direct route to chiral pyrimidine acyclic nucleoside analogues with a sulfur-containing side chain has been reported via enantioselective tandem conjugated addition/protonation reactions of thioacetic acid to α-pyrimidine substituted acrylates. In the presence of a quinine-derived thiourea catalyst, diverse chiral sulfur-containing pyrimidine acyclic nucleoside analogues were formed in excellent yields (95–99 %) and moderate to excellent enantioselectivities (up to 99 % ee).
中文翻译:

通过对映选择性串联共轭加成/质子合成具有硫侧链的手性无环嘧啶核苷。
AdPro:通过硫代乙酸与α-嘧啶取代的丙烯酸酯的对映选择性串联共轭加成/质子化反应,已经报道了具有含硫侧链的手性嘧啶无环核苷类似物的直接途径。在奎宁衍生的硫脲催化剂的存在下,形成了多种手性含硫嘧啶无环核苷类似物,收率极高(95–99%),对映选择性中等(至99%ee)。
更新日期:2017-11-22
中文翻译:

通过对映选择性串联共轭加成/质子合成具有硫侧链的手性无环嘧啶核苷。
AdPro:通过硫代乙酸与α-嘧啶取代的丙烯酸酯的对映选择性串联共轭加成/质子化反应,已经报道了具有含硫侧链的手性嘧啶无环核苷类似物的直接途径。在奎宁衍生的硫脲催化剂的存在下,形成了多种手性含硫嘧啶无环核苷类似物,收率极高(95–99%),对映选择性中等(至99%ee)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号