当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Determination of Activation Energy of Nucleophilic Aromatic Substitution on Porphyrins
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.jchemed.6b00940 Waqar Rizvi 1, 2 , Emaad Khwaja 1 , Saim Siddiqui 1 , N. V. S. Dinesh K. Bhupathiraju 1 , Charles Michael Drain 1, 3
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.jchemed.6b00940 Waqar Rizvi 1, 2 , Emaad Khwaja 1 , Saim Siddiqui 1 , N. V. S. Dinesh K. Bhupathiraju 1 , Charles Michael Drain 1, 3
Affiliation
|
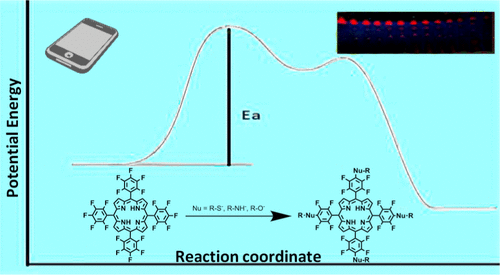
A physical organic chemistry experiment is described for second-year college students. Students performed nucleophilic aromatic substitution (NAS) reactions on 5,10,15,20-tetrakis(2,3,4,5,6-pentafluorophenyl)porphyrin (TPPF20) using three different nucleophiles. Substitution occurs preferentially at the 4-position (para) because it is thermodynamically favored, and the 2- and 6- (ortho) positions are kinetically disfavored because of steric interactions with the porphyrin ring. The activation energy depends heavily on the nucleophile. Open-source software (ImageJ from NIH) was used to quantify relative intensities of spots on a TLC plate obtained from different times and varying temperatures. These data were used to generate Arrhenius plots allowing students to determine relative activation energies for three different primary nucleophiles. The experiment was developed by 5 undergraduates and evaluated by 40 organic chemistry II students and 8 students in a physical chemistry laboratory. Students gained a deeper understanding of the relationships between the NAS mechanism, Arrhenius plots, and activation energy.
中文翻译:

卟啉亲核芳香取代活化能的实验测定
描述了针对二年级大学生的物理有机化学实验。学生使用三种不同的亲核试剂对5,10,15,20-四(2,3,4,5,6-五氟苯基)卟啉(TPPF 20)进行了亲核芳香取代(NAS)反应。取代优先发生在4位(对位),因为它在热力学上受到青睐,而2位和6位(邻位))位置由于与卟啉环的空间相互作用而在动力学上不利。活化能在很大程度上取决于亲核试剂。使用开源软件(来自NIH的ImageJ)来量化从不同时间和不同温度获得的TLC板上斑点的相对强度。这些数据用于生成Arrhenius图,使学生能够确定三种不同的主要亲核试剂的相对活化能。该实验由5位本科生开发,并由40位有机化学II学生和8位学生在物理化学实验室进行了评估。学生对NAS机制,Arrhenius图和激活能之间的关系有了更深入的了解。
更新日期:2017-11-22
中文翻译:

卟啉亲核芳香取代活化能的实验测定
描述了针对二年级大学生的物理有机化学实验。学生使用三种不同的亲核试剂对5,10,15,20-四(2,3,4,5,6-五氟苯基)卟啉(TPPF 20)进行了亲核芳香取代(NAS)反应。取代优先发生在4位(对位),因为它在热力学上受到青睐,而2位和6位(邻位))位置由于与卟啉环的空间相互作用而在动力学上不利。活化能在很大程度上取决于亲核试剂。使用开源软件(来自NIH的ImageJ)来量化从不同时间和不同温度获得的TLC板上斑点的相对强度。这些数据用于生成Arrhenius图,使学生能够确定三种不同的主要亲核试剂的相对活化能。该实验由5位本科生开发,并由40位有机化学II学生和8位学生在物理化学实验室进行了评估。学生对NAS机制,Arrhenius图和激活能之间的关系有了更深入的了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号