当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Haptacyclic Carbazole-Based Ladder-Type Nonfullerene Acceptor with Side-Chain Optimization for Efficient Organic Photovoltaics
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acsami.7b12612 Yu-Tang Hsiao,Chia-Hua Li,Shao-Ling Chang,Soowon Heo,Keisuke Tajima,Yen-Ju Cheng,Chain-Shu Hsu
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acsami.7b12612 Yu-Tang Hsiao,Chia-Hua Li,Shao-Ling Chang,Soowon Heo,Keisuke Tajima,Yen-Ju Cheng,Chain-Shu Hsu
|
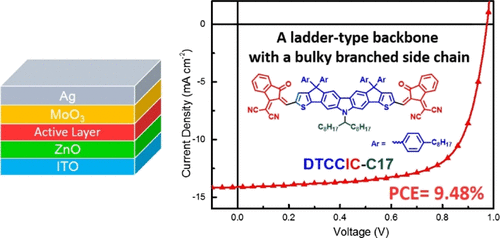
In this research, a haptacyclic carbazole-based dithienocyclopentacarbazole (DTCC) ladder-type structure was formylated to couple with two 1,1-dicyanomethylene-3-indanone (IC) moieties, forming a new nonfullerene acceptor DTCCIC-C17 using a bulky branched 1-octylnonayl side chain at the nitrogen of the embedded carbazole and four 4-octylphenyl groups at the sp3-carbon bridges. The rigid and coplanar main-chain backbone of the DTCC core provides a broad light-absorbing window and a higher-lying LUMO energy level, whereas the bulky flanked side chains reduce intermolecular interactions, making DTCCIC-C17 amorphous with excellent solution processability. The DTCCIC-C17 as an acceptor is combined with a medium band gap polymer poly[(2,6-(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)-benzo[1,2-b:4,5-b′]dithiophene))-alt-(5,5-(1′,3′-di-2-thienyl-5′,7′-bis(2-ethylhexyl)benzo[1′,2′-c:4′,5′-c′]dithiophene-4,8-dione))] (PBDB-T) as the donor in the active layer to obtain suitable highest occupied molecular orbital/lowest unoccupied molecular orbital energy alignments and complimentary absorption. The devices with an inverted configuration (ITO/ZnO/active layer/MoO3/Ag) without using an aqueous poly(3,4-ethylenedioxythiophene) polystyrene sulfonate layer were fabricated for better device stability. When the diiodooctane-treated PBDB-T:DTCCIC-C17 active layer was thermally annealed at 50 °C for 10 min, the device achieved the highest efficiency of 9.48% with a high Voc of 0.98 V, a Jsc of 14.27 mA cm–2, and an FF of 0.68.
中文翻译:

高效连锁的基于杂环的咔唑基梯形非富勒烯受体的侧链优化
在这项研究中,基于四环咔唑的二硫代环戊碳咔唑(DTCC)梯型结构被甲酰化,与两个1,1-二氰亚甲基-3-茚满酮(IC)部分偶联,形成了一个新的非富勒烯受体DTCCIC-C17,其使用了大分子支链1在嵌入的咔唑的氮原子上具有-辛基壬基侧链,在sp 3-碳桥处具有四个4-辛基苯基基团。DTCC核的刚性且共面的主链主链提供了宽广的光吸收窗口和更高的LUMO能级,而庞大的侧翼侧链减少了分子间的相互作用,使DTCCIC-C17非晶态并具有出色的溶液可加工性。DTCCIC-C17作为受体与中带隙聚合物聚[(2,6-(4,8-双(5-(2-乙基己基)噻吩-2-基)-苯并[1,2- b]:4,5- b ']二噻吩))- alt-(5,5-(1',3'-二-2-噻吩基-5',7'-双(2-乙基己基)苯并[1',2 '' -c:4',5'- c '] dithiophene-4,8-dione))](PBDB-T)作为活性层中的供体,以获得合适的最高占据分子轨道/最低最低未占据分子轨道能量排列和补充吸收。为了获得更好的装置稳定性,制造了不使用含水聚(3,4-乙撑二氧噻吩)聚苯乙烯磺酸盐层的具有反转结构(ITO / ZnO /活性层/ MoO 3 / Ag)的装置。当二碘辛烷处理过的PBDB-T:DTCCIC-C17活性层在50°C下热退火10分钟时,该器件在高V oc的情况下达到了9.48%的最高效率。0.98 V的Jsc,14.27 mA cm –2的J sc和0.68的FF。
更新日期:2017-11-22
中文翻译:

高效连锁的基于杂环的咔唑基梯形非富勒烯受体的侧链优化
在这项研究中,基于四环咔唑的二硫代环戊碳咔唑(DTCC)梯型结构被甲酰化,与两个1,1-二氰亚甲基-3-茚满酮(IC)部分偶联,形成了一个新的非富勒烯受体DTCCIC-C17,其使用了大分子支链1在嵌入的咔唑的氮原子上具有-辛基壬基侧链,在sp 3-碳桥处具有四个4-辛基苯基基团。DTCC核的刚性且共面的主链主链提供了宽广的光吸收窗口和更高的LUMO能级,而庞大的侧翼侧链减少了分子间的相互作用,使DTCCIC-C17非晶态并具有出色的溶液可加工性。DTCCIC-C17作为受体与中带隙聚合物聚[(2,6-(4,8-双(5-(2-乙基己基)噻吩-2-基)-苯并[1,2- b]:4,5- b ']二噻吩))- alt-(5,5-(1',3'-二-2-噻吩基-5',7'-双(2-乙基己基)苯并[1',2 '' -c:4',5'- c '] dithiophene-4,8-dione))](PBDB-T)作为活性层中的供体,以获得合适的最高占据分子轨道/最低最低未占据分子轨道能量排列和补充吸收。为了获得更好的装置稳定性,制造了不使用含水聚(3,4-乙撑二氧噻吩)聚苯乙烯磺酸盐层的具有反转结构(ITO / ZnO /活性层/ MoO 3 / Ag)的装置。当二碘辛烷处理过的PBDB-T:DTCCIC-C17活性层在50°C下热退火10分钟时,该器件在高V oc的情况下达到了9.48%的最高效率。0.98 V的Jsc,14.27 mA cm –2的J sc和0.68的FF。











































 京公网安备 11010802027423号
京公网安备 11010802027423号