当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into Azetidine Polymerization for the Preparation of Poly(propylenimine)-Based CO2 Adsorbents
Macromolecules ( IF 5.5 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.macromol.7b02402 Michele L. Sarazen 1 , Christopher W. Jones 1
Macromolecules ( IF 5.5 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.macromol.7b02402 Michele L. Sarazen 1 , Christopher W. Jones 1
Affiliation
|
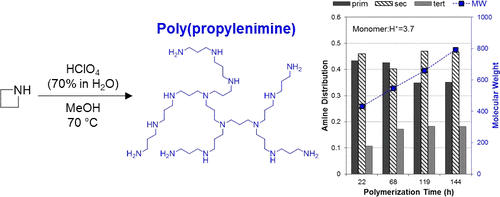
The cationic ring-opening polymerization of azetidine to form branched poly(propylenimine) (PPI) is investigated for the purpose of evaluating the utility of PPI/silica composite adsorbents for CO2 capture. The polymerization kinetics and primary:secondary:tertiary amine distribution are monitored with 1H NMR during reaction with varied synthesis conditions (i.e., reaction time 20–150 h), temperature (343–353 K), and monomer to acid initiator (here, HClO4) ratio. It is found that primary amines are converted to tertiary amines with increased polymerization time, while the addition of monomer over the first 6 h of polymerization increases the primary amine content. This suggests a mechanism where the monomer is rapidly consumed, leaving dimers or small oligomers that still contain rings as key reaction centers. The synthesized polymer is neutralized with either NH4OH or a basic resin and impregnated into mesoporous silica (SBA-15). The CO2 capture properties of these composite adsorbents are investigated, giving information about the effectiveness of the acid neutralization processes.
中文翻译:

氮杂环丁烷聚合制备基于聚丙二胺的CO 2吸附剂的见解
为了评估PPI /二氧化硅复合吸附剂在CO 2捕集中的用途,研究了氮杂环丁烷的阳离子开环聚合反应以形成支链聚丙二胺(PPI)。聚合反应动力学和伯:仲:叔胺分布与监测1具有不同的合成条件(即,反应时间20-150 h)中,温度(343-353 K),和单体酸引发剂(在此反应期间H NMR,盐酸4) 比率。发现随着增加的聚合时间,伯胺被转化为叔胺,而在聚合的前6小时内单体的添加增加了伯胺的含量。这表明单体被快速消耗的机制,从而留下仍含有环作为关键反应中心的二聚体或小的低聚物。合成的聚合物用NH 4 OH或碱性树脂中和,并浸渍到中孔二氧化硅(SBA-15)中。对这些复合吸附剂的CO 2捕集性能进行了研究,从而给出了有关酸中和过程有效性的信息。
更新日期:2017-11-21
中文翻译:

氮杂环丁烷聚合制备基于聚丙二胺的CO 2吸附剂的见解
为了评估PPI /二氧化硅复合吸附剂在CO 2捕集中的用途,研究了氮杂环丁烷的阳离子开环聚合反应以形成支链聚丙二胺(PPI)。聚合反应动力学和伯:仲:叔胺分布与监测1具有不同的合成条件(即,反应时间20-150 h)中,温度(343-353 K),和单体酸引发剂(在此反应期间H NMR,盐酸4) 比率。发现随着增加的聚合时间,伯胺被转化为叔胺,而在聚合的前6小时内单体的添加增加了伯胺的含量。这表明单体被快速消耗的机制,从而留下仍含有环作为关键反应中心的二聚体或小的低聚物。合成的聚合物用NH 4 OH或碱性树脂中和,并浸渍到中孔二氧化硅(SBA-15)中。对这些复合吸附剂的CO 2捕集性能进行了研究,从而给出了有关酸中和过程有效性的信息。



























 京公网安备 11010802027423号
京公网安备 11010802027423号